

Received 2024-12-18
Revised 2025-06-07
Accepted 2025-07-18
Prevalence of Interstitial Lung Disease in Patients with Primary Sjogren’s Syndrome: A Systematic Review and Meta-analysis of Observational
Studies
Majid Molahoseini 1, Mansour Salesi 1
1 Isfahan University of Medical Science, Isfahan, Iran
|
Abstract Background: Interstitial Lung Disease (ILD) is the most common pulmonary manifestation in patients with primary Sjögren’s syndrome. Therefore, this study aimed to investigate the prevalence of ILD in patients with primary Sjögren’s syndrome (pSS) using a systematic review and meta-analysis method. Materials and Methods: This study searched databases ProQuest, PubMed, Web of Science, Cochrane, Embase, and the search engine Google Scholar until February 8, 2025. Studies were combined using the sample size and variance of each study. The heterogeneity of the studies was examined using the Q Cochrane test and the I2 index. The significance level of the tests was considered P<0.05. Results: 34 observational studies, with a total of 9535 individuals, showed that the prevalence of ILD in all patients with pSS was 23.5% (95%CI: 18.6%, 28.4%), in women 31.2% (95%CI: 18.4%, 44.1%), and in men 45.5% (95%CI: 23.6%, 67.4%). Moreover, the prevalence of ILD in patients with pSS in the age group of 30 to 39 years was 22.2% (95%CI: 18.9%, 26%), 40 to 49 years 10.8% (95%CI: 8.7%, 12.9%), 50 to 59 years 27.6% (95%CI: 17.9%, 37.4%), and 60 to 69 years 23.3% (95%CI: 17.9%, 28.7%). Also, the prevalence of ILD in patients with pSS in case-control studies was 15.6% (95%CI: 7.1%, 24.1%), in cohort studies 25.7% (95%CI: 19%, 32.4%), and in cross-sectional studies 21.4% (95%CI: 11.9%, 31%). However, the prevalence of non-specific interstitial pneumonia (NSIP) was 48.8% (95%CI: 41.4%, 56.2%), usual interstitial pneumonia (UIP) 15% (95%CI: 10.5%, 19.5%), LIP 10.8% (95%CI: 6.6%, 15%), and organizing pneumonia (OP) 9.4% (95%CI: 5.4%, 13.4%). Conclusion: One out of every four patients with pSS has ILD, and the prevalence of ILD was higher in men than in women. Moreover, the most common type of ILD was NSIP, followed by UIP, OP, and LIP, and the highest prevalence of ILD was in the age group of 50 to 59 years. Evaluating further studies regarding the impact of various factors on ILD can help in better diagnosing it in patients. [GMJ.2025;14:e3752] DOI:3752 Keywords: Primary Sjogren’s Syndrome; Primary Sicca Syndrome; Prevalence; Lung Diseases; Interstitial; Interstitial Lung Disease; Interstitial Pneumonia |
|
GMJ Copyright© 2025, Galen Medical Journal. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/) Email:gmj@salviapub.com |

|
Correspondence to: Mansour Salesi, Isfahan University of Medical Science, Isfahan, Iran. Telephone Number:+989131210325 Email Address: mansour1380@yahoo.com |
|
GMJ.2025;14:e3752 |
www.salviapub.com
|
Molahoseini M, et al. |
Prevalence of Interstitial Lung Disease in Patients with Primary Sjogren’s Syndrome |
|
2 |
GMJ.2025;14:e3752 www.gmj.ir |
Introduction
Primary Sjögren’s syndrome (pSS) is a common systemic autoimmune disease characterized by chronic inflammation and dysfunction of the salivary and lacrimal glands [1, 2]. In this disease, the patient faces many personal and social issues. Therefore, it can affect the mental health of the patient and the social health of the society. For this reason, despite the high costs incurred for the diagnosis and treatment of the disease, it can cause a decrease in the quality of life and depression in patients [3]. In pSS, extra glandular organ systems such as lungs, kidneys, small vessels, and other endocrine glands are also implicated [4]. Sjögren’s syndrome is associated with many diseases, including an increased risk of non-Hodgkin’s lymphoma[5]. But regarding extra glandular manifestations, pulmonary complications rank at the top as having a higher prevalence in pSS compared to secondary type [6, 7], ranging from 9-75% according to the diagnostic method used, including small airway disease, parenchymal alteration, and interstitial involvement [8].
Among the pulmonary manifestations of pSS, interstitial lung disease (ILD) is by far the most common [9-11], with prevalences ranging between 8% and 39.1%. ILD causes airway obstruction in patients [12, 13]. Various factors contribute to the development of ILD [14]. Differences in prevalence can be due to various factors, including diagnostic criteria, environmental factors, race, the presence of underlying disease, and other factors [15-17]. The most frequently encountered subtype of interstitial lung disease is non-specific interstitial pneumonia (NSIP), whereas other sorts are usual interstitial pneumonia (UIP), lymphocytic interstitial pneumonia (LIP), and organizing pneumonia (OP) [18-21]. Interstitial lung disease is a common extraglandular complication related to survival in patients with pSS, which lowers the quality of life of those affected and serves as one of the frequent causes of premature death [22-25]. Conversely, systemic autoimmune diseases have been postulated to increase the risk of interstitial lung disease as reported in previous studies [26]. The prevalence of ILD in pSS patients has been reported differently and has been studied in different populations with heterogeneous factors. Therefore, systematic reviews and meta-analyses can provide more accurate and reliable evidence on this issue. Given that limited studies have been conducted in this area, and systematic reviews can evaluate all factors affecting the frequency of ILD, we investigated this issue in this study.
Materials and Methods
Study Protocol
This study was designed to evaluate the prevalence of ILD in patients with pSS. The PRISMA protocol [27], which is specific for systematic review and meta-analysis studies, was used and registered on the PROSPERO website (CRD42024566698).
PICO Components
Population: Studies investigating the frequency or prevalence of ILD in patients with pSS were evaluated. Intervention: Not applicable. Comparison: Not applicable. Outcomes: The primary outcome was the prevalence of ILD in patients with pSS. Secondary outcomes included the prevalence of NSIP, UIP, LIP, and OP in patients with pSS.
Search Strategy
To access resources, international databases ProQuest, PubMed, Web of Science, Cochrane, Embase, and the search engine Google Scholar were searched until July 1, 2024, without any time and place restrictions, using keywords (Primary Sjogren’s Syndrome, Primary Sicca Syndrome, Prevalence, “Lung Diseases, Interstitial”, Interstitial Lung Disease, Interstitial Pneumonia) and their MeSH equivalents. Keywords were combined with operators (AND, OR), and an advanced search was performed. The list of resources of selected studies in the electronic search phase was manually reviewed. The search strategy in the PubMed database is mentioned in this section: ((Primary Sjogren's Syndrome OR Primary Sicca Syndrome) AND ("Lung Diseases, Interstitial" OR Interstitial Lung Disease OR Interstitial Pneumonia)) AND (Prevalence)
Inclusion and Exclusion Criteria
Studies that had investigated the prevalence of ILD in patients with pSS were included in the meta-analysis. The following studies were excluded from the meta-analysis: studies where the type of Sjögren’s disease was not specified - letters to the editor - case reports - duplicate studies - studies where their data were incomplete - review studies - studies that did not have full text - studies with poor quality.
Qualitative Assessment
Two authors evaluated the studies with the Newcastle Ottawa Scale checklist. In this checklist, a star system was used, such that a maximum of one star was given for each question, and only the question related to comparison had the possibility of allocating two stars. Therefore, the lowest score on the checklist was zero (lowest quality), and the highest score was ten (highest quality). The cut-off point in this study was considered six [28].
Data Extraction
Two authors independently performed data extraction, and disagreements were resolved by a third researcher. From each study, information such as author’s name, type of study, sample size, country, number of women and men, year, average age, prevalence of ILD in patients with pSS in all individuals, prevalence of ILD in women with pSS, prevalence of ILD in men with pSS, prevalence of ILD subgroups in patients with pSS was extracted.
Statistical Analysis
Metaprop implements procedures which are specific to binomial data and allows computation of exact binomial and score test-based confidence intervals. It provides appropriate methods for dealing with proportions close to or at the margins where the normal approximation procedures often break down, by use of the binomial distribution to model the within-study variability or by allowing Freeman-Tukey double arcsine transformation to stabilize the variances. Sensitivity analysis was also used to determine the most influential studies resulting from the current meta-analysis. Subgroup analysis and meta-regression were used to investigate the sources of heterogeneity, and the Funnel plot was used to assess publication bias. The I2 index has three classifications (<25% indicates low heterogeneity, between 25% and 75% shows moderate heterogeneity and >75% indicates high heterogeneity). Due to the moderate heterogeneity, this study applied a random-effects model. Data analysis was performed with STATA 14 software (developed by Computing Resource Center in California) , and the significance level of the tests was considered P<0.05.
Results
Study Selection
There was a total of 302 studies at the initial stage. The study titles were screened, and 142 duplicate studies were removed. Abstracts of studies were assessed, and we excluded 14 studies because they did not have full text. The full text of the remaining 61 articles was evaluated; then41 studies without essential data were excluded. Seven further studies were excluded based on other items from the exclusion criteria, and 34 articles entered the systematic review and meta-analysis (Figure-1). This meta-analysis included 34 observational studies involving a population sample of 9535 individuals. Of these 34 studies, 26 were cohort studies, 5 were case-control studies, and 3 were cross-sectional studies. The studies were published from 1985 to 2024 (Table-1).
When excluding the studies of Sahin Ozdemirel [40] and Kvarnstrom [54], which were considered outliers, the overall prevalence estimate for ILD in pSS patients was 23.5% (95%CI: 18.6%, 28.4%) (Figure-2A). Furthermore, the prevalence of ILD in women with pSS was 31.2% (95%CI: 18.4%, 44.1%), and in men was 45.5% (95%CI: 23.6%, 67.4%) (Figures-2B and -2C). Consequently, the incidence of ILD in male patients with pSS was higher than in female patients, and the male gender could be considered an independent risk factor for developing ILD. As shown in Figure-2D, the subgroup analysis according to age detected a prevalence of ILD in patients with pSS:
• Age 30-39 years: 22.2% (95%CI: 18.9%, 26%)
• Age 40-49 years: 10.8% (95%CI: 8.7%, 12.9%)
• Age 50-59 years: 27.6% (95%CI: 17.9%, 37.4%)
• Age 60-69 years: 23.3% (95%CI: 17.9%, 28.7%)
Age was not statistically significantly associated with pSS-associated ILD, and the highest prevalence of ILD was in the age group of 50 to 59 years.
Regarding study design, the prevalence of ILD among pSS patients in:
• Case-control studies was 15.6% (95%CI: 7.1%, 24.1%)
• Cohort studies was 25.7% (95%CI: 19%, 32.4%)
• Cross-sectional studies was 21.4% (95%CI: 11.9%, 31%) (Figure-2E)
In patients with pSS, the prevalence of NSIP was 48.8% (95%CI: 41.4%, 56.2%), the prevalence of UIP was 15% (95%CI: 10.5%, 19.5%), the prevalence of LIP was 10.8% (95%CI: 6.6%, 15%), and the prevalence of OP was 9.4% (95%CI: 5.4%, 13.4%). Therefore, the most common type of ILD in patients with pSS, in order, was NSIP, UIP, OP, and LIP (Figures-3A-D). Figure-4 shows a meta-regression, indicating there was no statistically significant relationship between the prevalence of ILD in patients with pSS and the sample size of the studies (P=0.608). Moreover, Figure-5 demonstrates that the relationship between the prevalence of ILD in patients with pSS and the year of publication of the studies was not statistically significant (P=0.841). The publication bias diagram was significant (P=<0.001) and showed that studies that reported a high prevalence of ILD in patients with primary Sjögren's syndrome had a lower chance of publication (Figure-6). Sensitivity analysis showed that the studies by Wang Y (2018) and Kvarnstrom M (2015) were the most influential studies in the current research results (Figure-7).
Discussion
In this meta-analysis, 34 observational studies were combined, and a total of 9535 patients with pSS were examined. The results revealed that the prevalence of ILD in all patients with pSS was 23.5%, and in women and men, it was 31.2% and 45.5%, respectively. Moreover, the prevalence of ILD in patients aged 30 to 39 years was 22.2%, 40 to 49 years was 10.8%, 50 to 59 years was 27.6%, and 60 to 69 years was 23.3%. In terms of types of ILD, the prevalence of NSIP in patients was 48.8%, UIP was 15%, LIP was 10.8%, and OP was 9.4%. In the current meta-analysis, NSIP was the most common type of ILD. In another study, the authors estimated a combined prevalence of 52% for NSIP and 44% for UIP in patients with pSS [60], which is consistent with the results of our study.
In 2024, in a cohort study conducted by Manikuppam and colleagues, which included 550 patients with pSS with an average age of 50 years, the prevalence of ILD was estimated to be 6%. This study was retrospective and the average age of the patients was close to 50 years. On the other hand, all patients were evaluated from one center. Therefore, these factors could be influential in the discrepancy between the results and the results of the present study [29].
In a study conducted by Lin and colleagues in 2022 on 333 patients with pSS, a retrospective study showed that the prevalence of ILD was 19.82%. In this study, it was found that the prevalence of ILD was higher in Asian populations than in Europeans. In contrast, in the present study, Iranian races were evaluated [35]. In another cohort study conducted by Guo and colleagues in 2021 on a large Chinese group consisting of 563 patients with pSS and 172 patients with secondary Sjögren’s syndrome, the prevalence of ILD in patients with pSS was 42.6% [38]. In a case-control study conducted by Gao and colleagues in 2018 on 853 patients with pSS, the aim of which was to investigate the prevalence, risk factors, and prognosis of ILD in patients with pSS, ILD was observed in 165 patients (19.34%) [19]. In another study conducted by Huang and colleagues in 2023 using a case-control method, the analysis of 274 patients with pSS showed that the prevalence of ILD was 22.3% [32]. Furthermore, due to the wide range of prevalence of ILD in patients with pSS, a systematic review and meta-analysis was required to perform. In some studies, the prevalence of ILD in patients with pSS was low, but on the other hand, in some studies, we faced a high prevalence of ILD in patients with pSS.
The difference in the prevalence of ILD in different studies compared to the present study could be influenced by various factors. These factors include geographical regions, race, age of patients, type of study, diagnostic criteria, and other factors. Therefore, factors can influence the frequency of ILD in different studies.
In a meta-analysis conducted in 2022 by Arbiv and colleagues to investigating the prevalence and radiological patterns of ILD in Sjögren’s syndrome, the prevalence of ILD was about 15% [61]. In the current meta-analysis, in line with the previous study, the prevalence of ILD in patients with pSS was 25. Although in the current study, only patients with pSS were examined, while in the previous study, patients with primary and secondary Sjögren’s were examined together.
In a systematic review conducted in 2020 by Sambataro and colleagues, the results showed that about 20% of pSS patients have ILD [62]. In a meta-analysis conducted by He and colleagues in 2020, in 23 studies with 6157 patients, the prevalence of ILD in patients with pSS was 13% [18]. Another meta-analysis by Joy and colleagues in 2023 aimed to investigate the prevalence, imaging patterns, and risk factors of ILD in connective tissue disease, and the prevalence of ILD in patients with pSS was 17% [63]. In 2023, in a meta-analysis conducted by Berardicurti and colleagues to investigate the prevalence of ILD in pSS, the combined prevalence of pSS-ILD was 23% [60]. In the current meta-analysis, which is the most up-to-date meta-analysis conducted in this field, the prevalence of ILD in patients with pSS has increased compared to previous meta-analyses, and considering the impact that ILD has on the quality of life and mortality of patients, this issue is very concerning.
Strengths of the current study include: )1) In the previous meta-analysis (Berardicurti), the databases PubMed, Embase, and Cochrane were searched until December 2022. While in the current meta-analysis, the databases ProQuest, PubMed, Web of Science, Cochrane, and Embase were searched until July 2024. )2) The number of studies and sample size in the current meta-analysis were greater than the number of studies and sample size in the previous meta-analysis (Berardicurti). )3) the prevalence of ILD in patients with pSS was presented by age and gender of patients, which was not present in the previous meta-analysis (Berardicurti). )4) the prevalence of NSIP and UIP, which were presented in the previous meta-analysis, the prevalence of LIP and OP was also reported in patients with pSS. )5) the results of the prevalence estimate of ILD in patients with pSS were reported by type of studies to reduce heterogeneity.
Conclusion
One in four patients with pSS has ILD. Since ILD has degrees and is in mild and asymptomatic stages, its identification is not easy, even this prevalence may be higher. On the other hand, the prevalence of ILD was higher in men than in women. For this reason, men with pSS are more at risk of ILD than women. Also, the prevalence of ILD in the age group of 50 to 59 years was higher than other groups. In addition, the most common type of ILD is NSIP, and almost one in two people is affected by NSIP. As you can see, the statistics are very high and worrying. Therefore, it is recommended that patients with pSS, especially men and people who are 50 to 59 years old, be screened for ILD, as they are considered a high-risk group.
Conflict of Interest
The authors declare that they have no conflict of interest.
|
Prevalence of Interstitial Lung Disease in Patients with Primary Sjogren’s Syndrome |
Molahoseini M, et al. |
|
GMJ.2025;14:e3752 www.gmj.ir |
3 |
|
Molahoseini M, et al. |
Prevalence of Interstitial Lung Disease in Patients with Primary Sjogren’s Syndrome |
|
4 |
GMJ.2025;14:e3752 www.gmj.ir |
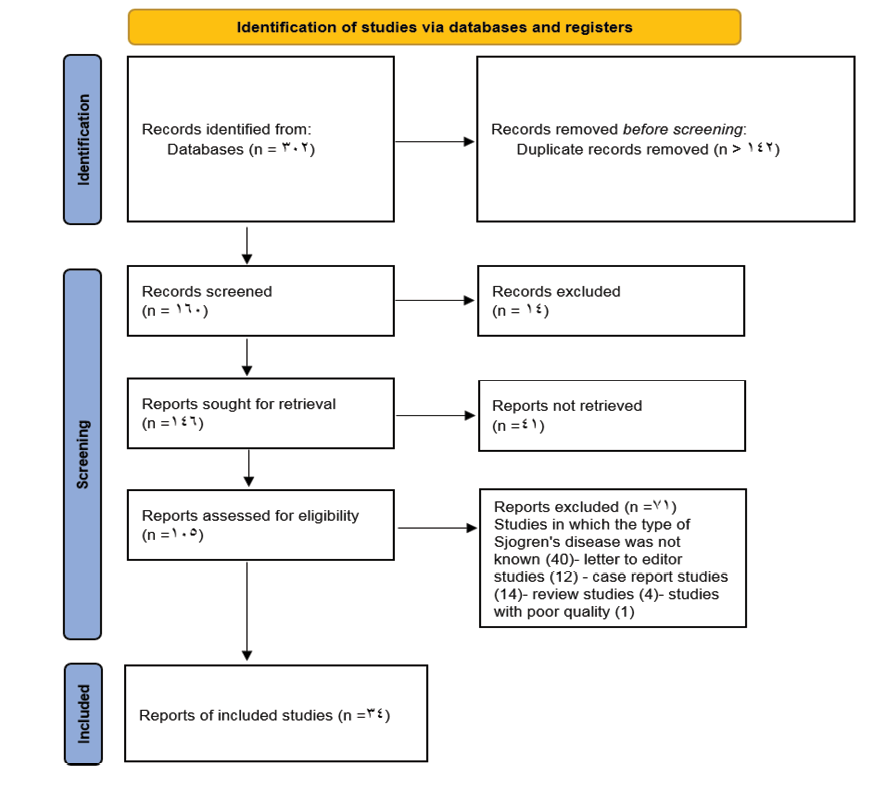
Figure 1. Flowchart of entering studies into the process of systematic review and meta-analysis
|
Prevalence of Interstitial Lung Disease in Patients with Primary Sjogren’s Syndrome |
Molahoseini M, et al. |
|
GMJ.2025;14:e3752 www.gmj.ir |
5 |
Table 1. Characteristics of Articles
|
Author, Year |
Location |
Design |
Sample size |
Age group (year) |
Number of female |
Number of male |
Duration of study |
Prevalence of ILD in patients with pSS (%) |
Prevalence of ILD in female (%) |
Prevalence of ILD in male (%) |
Score of Newcastle Ottawa Scale |
|
Manikuppam P, 2024[29] |
India |
Cohort |
550 |
50 |
NR |
NR |
From 2010 to 2019 |
6 |
NR |
NR |
9 |
|
Yang Z, 2024[30] |
China |
Cohort |
256 |
63.36 |
240 |
16 |
From October 2017 to January 2022 |
39.8 |
40.8 |
25 |
8 |
|
Wang Y, 2024[31] |
China |
Case-control |
622 |
NR |
NR |
NR |
NR |
18.8 |
NR |
NR |
8 |
|
Huang Y, 2023[32] |
China |
Case-control |
274 |
NR |
NR |
NR |
From August 2013 to August 2022 |
22.3 |
NR |
NR |
9 |
|
Lee KA, 2023[33] |
Korea |
Cohort |
120 |
60.35 |
107 |
13 |
Between 2013 and 2021 |
32.5 |
29.9 |
53.8 |
8 |
|
He SH, 2022[34] |
China |
Cohort |
83 |
NR |
NR |
NR |
NR |
18.9 |
NR |
NR |
7 |
|
Lin W, 2022[35] |
China |
Cohort |
333 |
54 |
310 |
23 |
From September 2016 to March 2019 |
19.8 |
18.7 |
34.7 |
8 |
|
Isik OO, 2022[36] |
Turkey |
Cross-sectional |
120 |
55.9 |
NR |
NR |
Between 2004 and 2019 |
13.3 |
NR |
NR |
7 |
|
Weng L, 2022[37] |
China |
Cohort |
69 |
55.04 |
NR |
NR |
Between September 2013 and June 2017 |
28 |
NR |
NR |
7 |
|
Guo T, 2021[38] |
China |
Cohort |
563 |
53 |
508 |
55 |
From January 2010 to May 2020 |
42.6 |
40.9 |
58.2 |
9 |
|
Continued on the next page |
|||||||||||
|
Molahoseini M, et al. |
Prevalence of Interstitial Lung Disease in Patients with Primary Sjogren’s Syndrome |
|
6 |
GMJ.2025;14:e3752 www.gmj.ir |
|
Continue of Table 1. Characteristics of Articles |
|||||||||||
|
Zhang K, 2021[39] |
China |
Cohort |
217 |
57.83 |
NR |
NR |
From May 2009 to November 2020 |
32.7 |
NR |
NR |
7 |
|
Sahin Ozdemirel T, 2021[40] |
Turkey |
Cohort |
35 |
54.4 |
NR |
NR |
Between September 2015 and December 2018 |
2.9 |
NR |
NR |
7 |
|
Yazisiz V, 2020[41] |
Turkey |
Cohort |
372 |
60.6 |
341 |
31 |
Between 2004 and 2014 |
12.6 |
9.4 |
48.4 |
8 |
|
Goules AV, 2020[42] |
Greece and Italy |
Case-control |
379 |
≤ 35 |
NR |
NR |
Between May 1984 and May 2019 |
2.1 |
NR |
NR |
9 |
|
Goules AV, 2020[42] |
Greece and Italy |
Case-control |
293 |
≥65 |
NR |
NR |
Between May 1984 and May 2019 |
7.9 |
NR |
NR |
8 |
|
Zhao R, 2020[43] |
China |
Cross-sectional |
101 |
51.58 |
89 |
12 |
From October 2017 to July 2018 |
27.7 |
NR |
NR |
8 |
|
Heus A, 2020[44] |
Netherlands |
Cohort |
262 |
56 |
NR |
NR |
2015 |
57 |
NR |
NR |
8 |
|
Sogkas G, 2020[45] |
Germany |
Cohort |
31 |
58.9 |
NR |
NR |
Between April 2018 and February 2020 |
61 |
NR |
NR |
8 |
|
Shi L, 2020[46] |
China |
Case-control |
706 |
60.4 |
NR |
NR |
Between January 2015 and April 2019 |
23.8 |
NR |
NR |
8 |
|
Guisado-Vasco P, 2019[47] |
Spain |
Cross-sectional |
81 |
69 |
NR |
NR |
Between January 2017 and September 2018 |
25.4 |
NR |
NR |
9 |
|
Dong X, 2018[15] |
China |
Cohort |
527 |
59 |
462 |
65 |
From January 2011 to December 2017 |
39.1 |
38.5 |
43.1 |
8 |
|
Continued on the next page |
|||||||||||
|
Prevalence of Interstitial Lung Disease in Patients with Primary Sjogren’s Syndrome |
Molahoseini M, et al. |
|
GMJ.2025;14:e3752 www.gmj.ir |
7 |
|
Continue of Table 1. Characteristics of Articles |
|||||||||||
|
Gao H, 2018[19] |
China |
Case-control |
853 |
61.25 |
NR |
NR |
Between January 2003 and March 2012 |
19.3 |
NR |
NR |
9 |
|
Wang Y, 2018[48] |
China |
Cohort |
201 |
58.88 |
176 |
25 |
January 2012 to December 2014 |
78.6 |
76.1 |
96 |
7 |
|
Kakugawa T, 2018[49] |
Japan |
Cohort |
101 |
65 |
95 |
6 |
From April 2008 to December 2015 |
31.7 |
NR |
NR |
7 |
|
Kampolis CF, 2018[50] |
Greece |
Cohort |
384 |
62.8 |
363 |
21 |
From 1993 to 2016 |
13 |
NR |
NR |
7 |
|
Manfredi A, 2017[17] |
Italy |
Cohort |
77 |
59.5 |
NR |
NR |
January 2013 to December 2106 |
16.9 |
NR |
NR |
8 |
|
Ramirez Sepulveda JI, 2017[51] |
Sweden/Norway |
Cohort |
481 |
46.1 |
444 |
37 |
NR |
7.9 |
7 |
19 |
7 |
|
Ter Borg EJ, 2017[52] |
Netherlands |
Cohort |
149 |
55.4 |
NR |
NR |
From June 1991 until August 2015 |
11.4 |
NR |
NR |
8 |
|
Li X, 2015[53] |
China |
Cohort |
315 |
46.8 |
304 |
11 |
From January 1999 to September 2012 |
20.9 |
20.7 |
27.3 |
7 |
|
Kvarnstrom M, 2015[54] |
Sweden |
Cohort |
194 |
55.1 |
NR |
NR |
From 1 January 2007 to 31 December 2011 |
1 |
NR |
NR |
8 |
|
Ter Borg EJ, 2014[55] |
Netherlands |
Cohort |
83 |
54.3 |
NR |
NR |
From June 1991 until November 2011 |
7.2 |
NR |
NR |
8 |
|
Nannini C, 2013[56] |
USA |
Cohort |
105 |
58.1 |
NR |
NR |
1976–2005 |
10 |
NR |
NR |
9 |
|
Lin DF, 2010[57] |
China |
Cohort |
522 |
39 |
NR |
NR |
Between 1985 and 2006 |
22.2 |
NR |
NR |
9 |
|
Papathanasiou MP, 1986[58] |
Greece |
Cohort |
40 |
53.05 |
NR |
NR |
NR |
37.5 |
NR |
NR |
7 |
|
Constantopoulos SH, 1985[59] |
Greece |
Cohort |
36 |
52.5 |
NR |
NR |
NR |
25 |
NR |
NR |
7 |
|
Molahoseini M, et al. |
Prevalence of Interstitial Lung Disease in Patients with Primary Sjogren’s Syndrome |
|
8 |
GMJ.2025;14:e3752 www.gmj.ir |
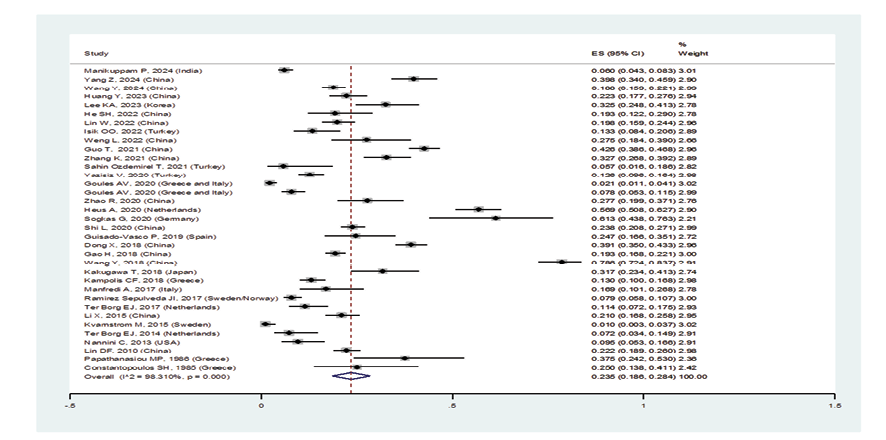
Figure 2 A. Forest plot related to the prevalence of ILD A: in patients with pSS and its 95% confidence interval
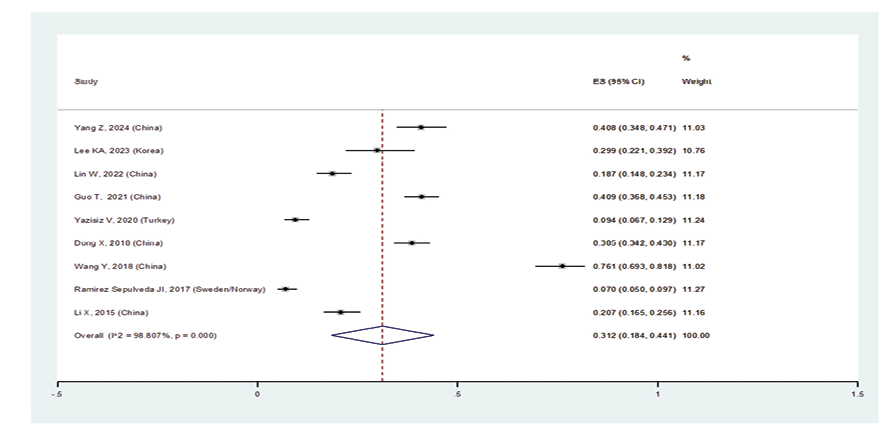
Figure 2 B. Forest plot related to the prevalence of ILD B: females with pSS
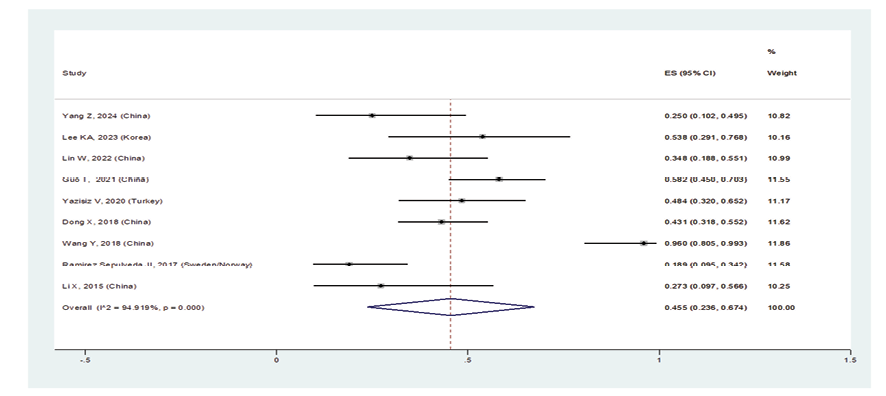
Figure 2 C. Forest plot related to the prevalence of ILD C: in males with pSS
|
Prevalence of Interstitial Lung Disease in Patients with Primary Sjogren’s Syndrome |
Molahoseini M, et al. |
|
GMJ.2025;14:e3752 www.gmj.ir |
9 |
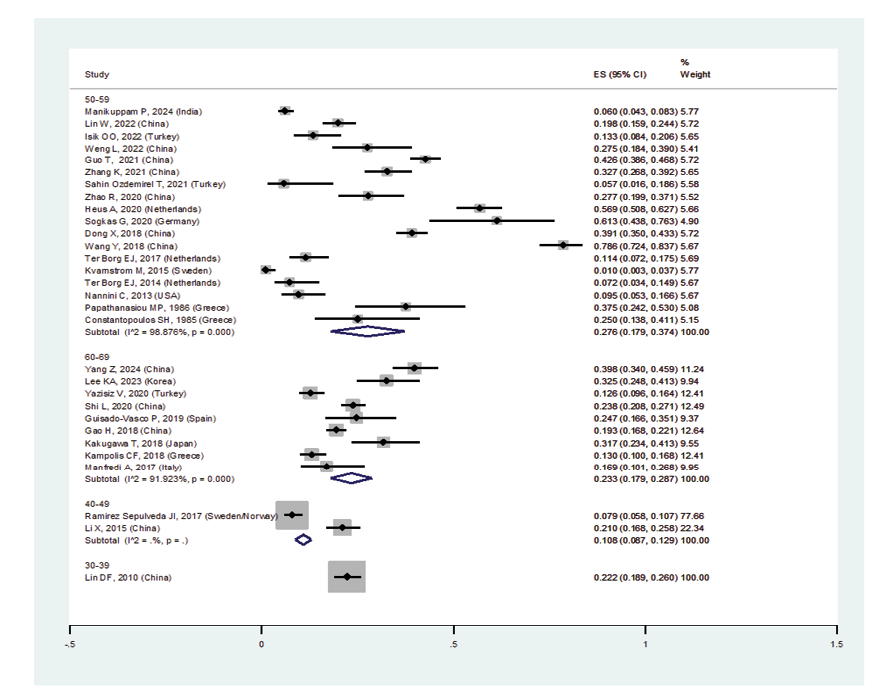
Figure 2 D. Forest plot related to the prevalence of ILD D: in patients with pSS by age group
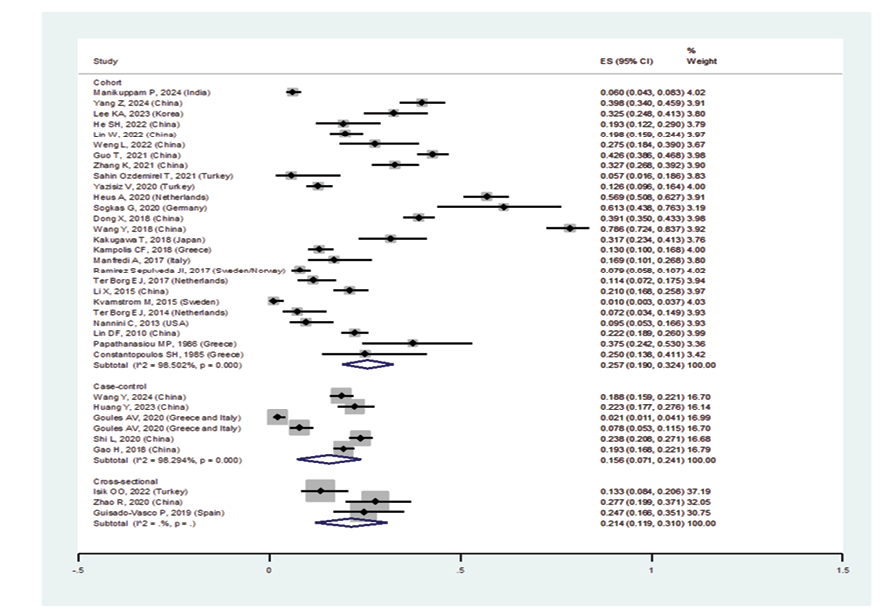
Figure 2 E. Forest plot related to the prevalence of ILD E: in patients with pSS by design.
|
Molahoseini M, et al. |
Prevalence of Interstitial Lung Disease in Patients with Primary Sjogren’s Syndrome |
|
10 |
GMJ.2025;14:e3752 www.gmj.ir |
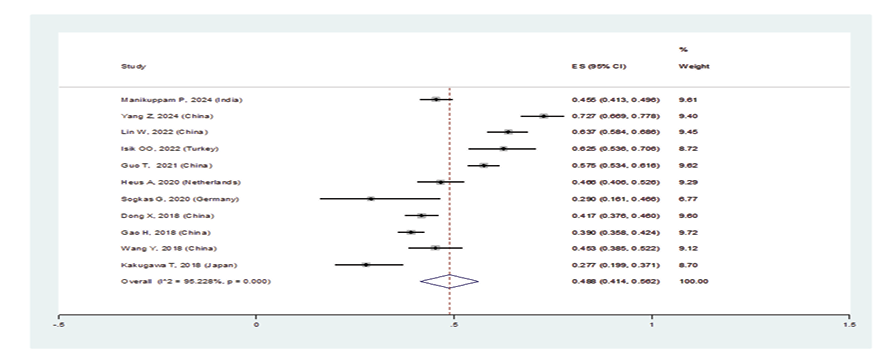
Figure 3 A. Forest plot related to the prevalence of A: nonspecific interstitial pneumonia in patients with pSS
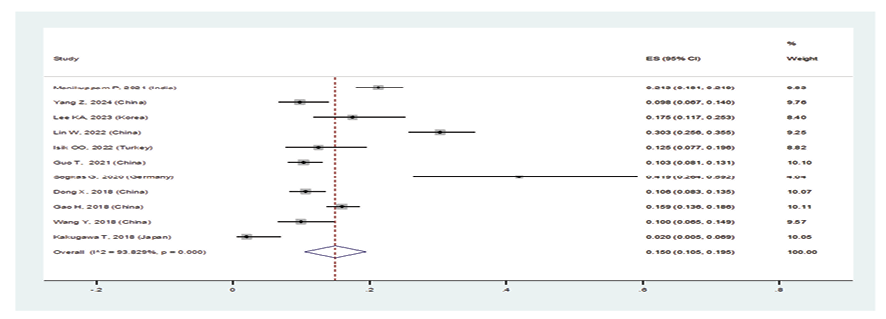
Figure 3 B. Forest plot related to the prevalence of B: usual interstitial pneumonia in patients with pSS
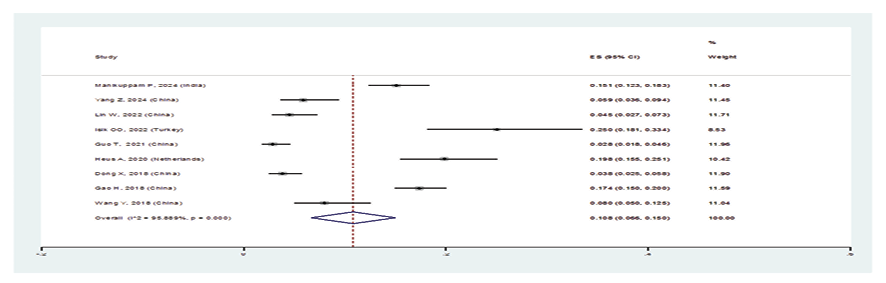
Figure 3 C. Forest plot related to the prevalence of C: lymphocytic interstitial pneumonia in patients with pSS
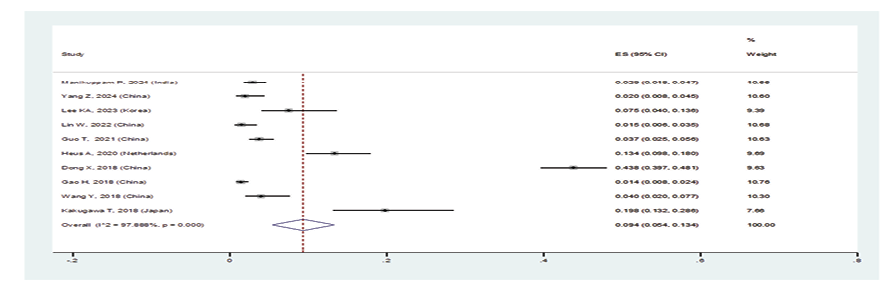
Figure 3 D. Forest plot related to the prevalence of D: organizing pneumonia in patients with pSS
|
Prevalence of Interstitial Lung Disease in Patients with Primary Sjogren’s Syndrome |
Molahoseini M, et al. |
|
GMJ.2025;14:e3752 www.gmj.ir |
11 |
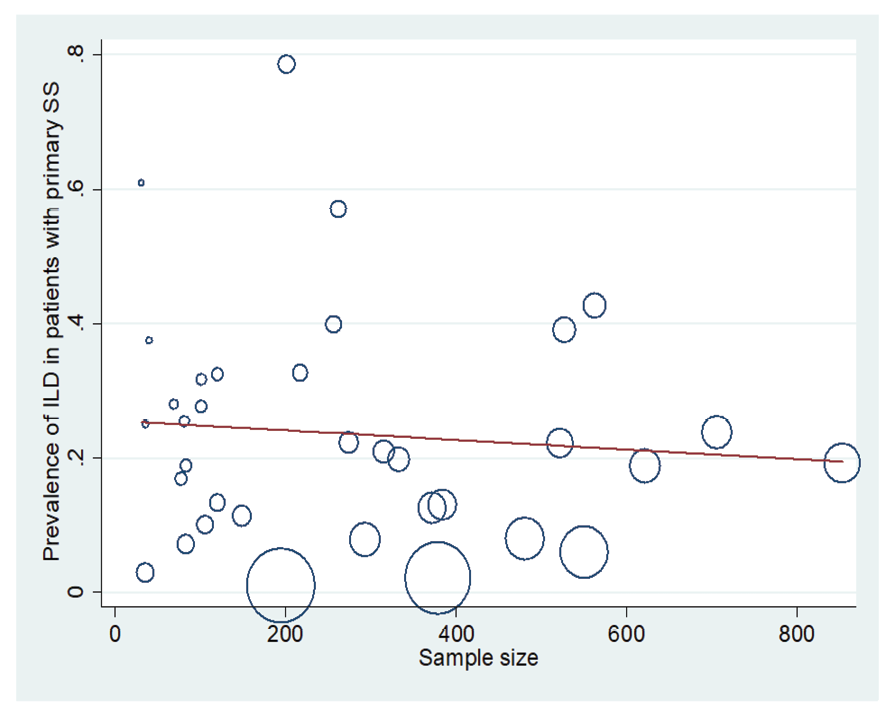
Figure 4. Meta-regression plot of the relationship between “prevalence of ILD in patients with pSS” and the sample size of studies
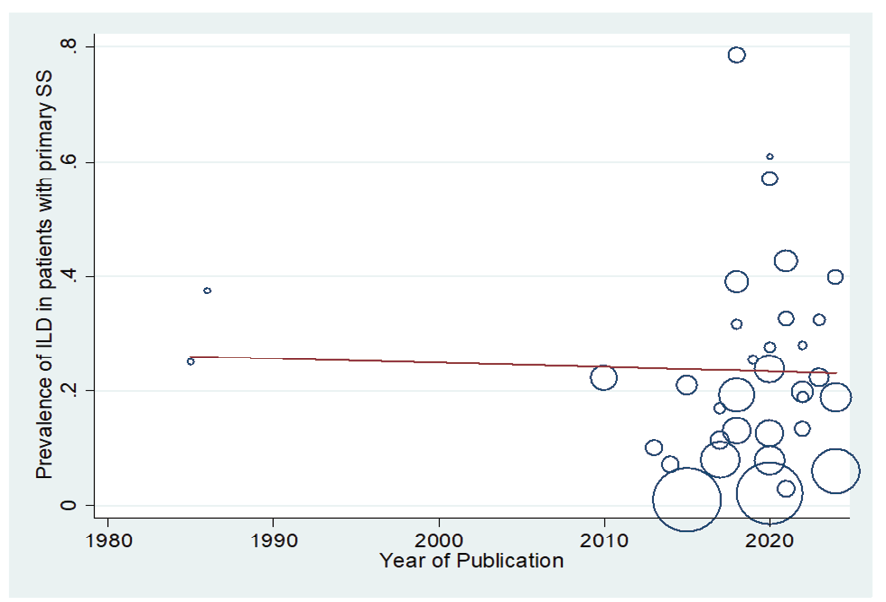
Figure 5. Meta-regression plot of the relationship between “prevalence of ILD in patients with pSS” and year of publication
|
Molahoseini M, et al. |
Prevalence of Interstitial Lung Disease in Patients with Primary Sjogren’s Syndrome |
|
12 |
GMJ.2025;14:e3752 www.gmj.ir |
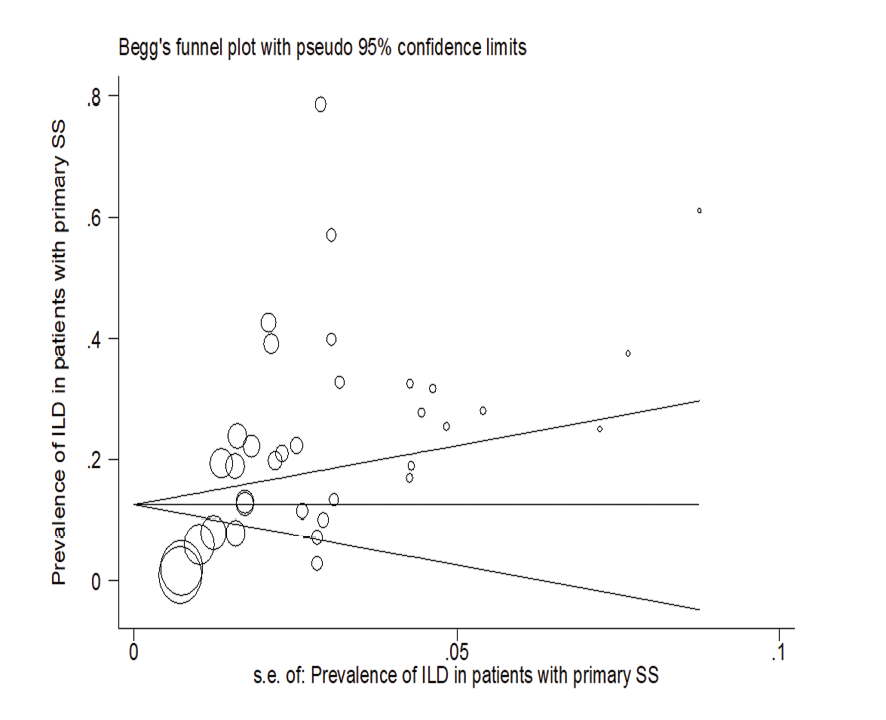
Figure 6. Publication bias
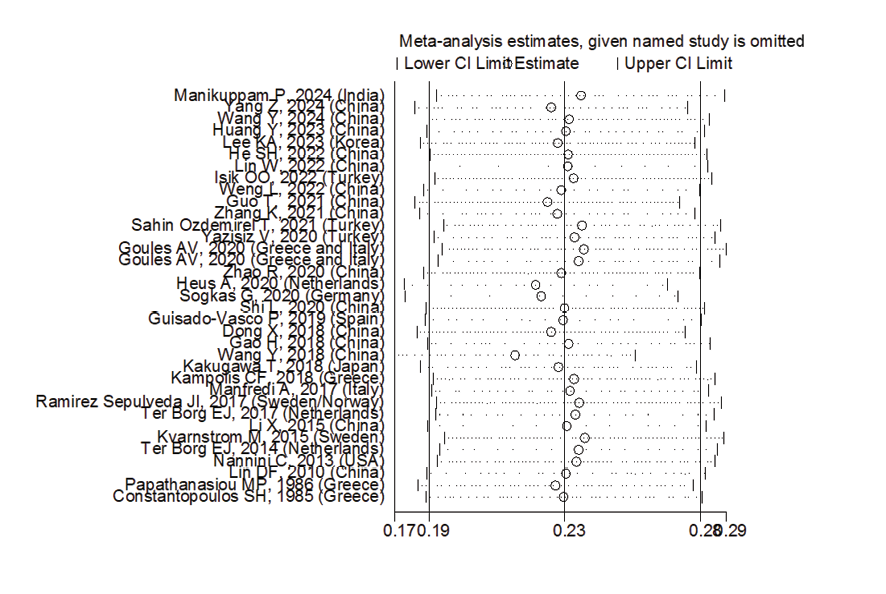
Figure 7. Sensitivity analysis
|
Prevalence of Interstitial Lung Disease in Patients with Primary Sjogren’s Syndrome |
Molahoseini M, et al. |
|
GMJ.2025;14:e3752 www.gmj.ir |
13 |
|
Molahoseini M, et al. |
Prevalence of Interstitial Lung Disease in Patients with Primary Sjogren’s Syndrome |
|
14 |
GMJ.2025;14:e3752 www.gmj.ir |
|
References |
|
Prevalence of Interstitial Lung Disease in Patients with Primary Sjogren’s Syndrome |
Molahoseini M, et al. |
|
GMJ.2025;14:e3752 www.gmj.ir |
15 |
|
Molahoseini M, et al. |
Prevalence of Interstitial Lung Disease in Patients with Primary Sjogren’s Syndrome |
|
16 |
GMJ.2025;14:e3752 www.gmj.ir |