

Received 2025-01-19
Revised 2025-04-16
Accepted 2025-06-10
Evaluation of the Association between Sjögren Syndrome and Thyroid Cancer Risk:
A Systematic Review and Meta-analysis
Mansour Salesi 1 , Shadi Botshekan 1
1 Isfahan University of Medical Science, Isfahan, Iran
|
Abstract Background: Sjögren’s syndrome affects the skin, joints, lungs, kidneys, liver, and thyroid. This research was aimed to assess the association of Sjögren’s syndrome with the thyroid carcinoma risk. Materials and Methods: The present study was used a systematic review and meta-analysis method. This study searched the databases ProQuest, PubMed, Web of Science, Cochrane, and the search engine Google Scholar until July 7, 2024. The level of significance was considered as P<0.05, and all data analyses were done in STATA 14 software. Results: A review of 11 studies revealed that Sjögren’s syndrome increased the thyroid carcinoma risk in all patients (OR: 2.08, (95%CI: 1.47, 2.94)), in patients aged 40 to 49 years (OR: 1.43, (95%CI: 1.23, 1.67)), 50 to 59 years (OR: 4.65, (95%CI: 1.87, 11.58)), 60 to 69 years (OR: 1.34, (95%CI: 1.08, 1.66)) and in women ((OR:1.83, (95%CI: 1.35, 2.48). However, there was no significant association between Sjögren’s syndrome and thyroid carcinoma risk in men ((OR: 1.49, (95%CI: 0.95, 2.34). Moreover, patients with Sjögren’s syndrome who had a follow-up period of <= 5 years ((OR: 1.68, (95%CI: 1.10, 2.54) and patients with a follow-up period of > 5 years ((OR: 5.77, (95%CI: 1.97, 16.97) were at risk of thyroid cancer. Moreover, the thyroid carcinoma risk was in Europe ((OR: 3.26, (95%CI: 1.24, 8.56) and in Asia ((OR: 1.87, (95%CI: 1.27, 2.74). Primary Sjögren’s syndrome also significantly increased the thyroid carcinoma risk ((OR: 2.37, (95%CI: 1.44, 3.90). Conclusion: Sjögren’s syndrome increased the thyroid carcinoma risk, and female gender, fifth decade of life, European race, and involvement duration of more than 5 years were the exacerbating factors. [GMJ.2025;14:e3753] DOI:3753 Keywords: Sjogren’s Syndrome; Sicca Syndrome; Thyroid Neoplasms; Thyroid Carcinoma; Thyroid Adenoma; Thyroid Cancer |
Introduction
It has been estimated that 0.5-1% of the world's population suffer from diagnosed Sjögren's syndrome [1]. Sjögren's syndrome is much more frequent in women than men, affecting mainly the annual 4th and 6th decade of life [2, 3]. Sjögren's syndrome is the prototypic exocrinopathy, defined by the infiltration of lymphocytes to exocrine glands causing substantial loss of secretory function and hence sicca symptoms (dry mouth and eyes) [4, 5]. Sjögren's syndrome presents symptoms of fatigue, xeroderma, cough due to dry naris and esophageal motility disorders, and neurological sequelae including peripheral neuropathy as well [6].
Sjögren's syndrome also attack the respiratory, digestive, urinary, circulatory, and nervous systems [7].
Another study stated that Sjögren's syndrome may be associated with the impairment of body parts, such as the skin, joints, muscles, and organs (lungs, kidneys, liver, and thyroid). These differences can be influenced by various conditions, including the type of study, the race of the patients, the population studied, and many other thing [8]. Previous studies have shown that the incidence of thyroid carcinoma in patients with Sjögren's syndrome is different. Thyroid carcinoma is one of the most common diseases seen in patients [9].
Thyroid carcinoma represents the predominant neoplasm within the endocrine system, and its prevalence has experienced a notable escalation. Korea has the highest proportion of thyroid tumors among all types of cancer incidence (45/100,000) worldwide [10, 11]. Patients with certain autoimmune diseases, such as rheumatoid arthritis (RA) [12], systemic lupus erythematosus (SLE) [13-15], and inflammatory bowel disease (IBD) [16, 17] are shown to have an increased thyroid carcinoma risk according to multiple meta-analyses and observational studies. Studies in Taiwan and Spain also conveyed an enhanced thyroid carcinoma risk in patients diagnosed with primary Sjogren's syndrome versus historical controls, which is compatible with our findings [18, 19].
Nevertheless, results from previous observational studies are conflicting. In some studies, no statistical association between Sjögren's syndrome and thyroid cancer was mentioned [20, 21], while in other works it is described as a significant risk factor for the development of this neoplasm [22, 23].
Despite growing interest in autoimmune-cancer links, no prior meta-analysis has focused on SjS and thyroid carcinoma. Consequently, this is the first meta-analysis to investigate an association between Sjögren's syndrome and thyroid neoplasm risk, taking into account the results from previous studies, thereby clarifying such a controversial point.
Materials and Methods
Study Protocol
The PRISMA protocol [24], specifically designed for systematic review and meta-analysis studies, was used for study design. This research methodology was adequately registered and documented on the PROSPERO platform (CRD42024569043).
PICO Components
Population: Without considering any research type, spatial, or temporal restrictions, all studies that investigated the association Sjögren's syndrome with thyroid carcinoma risk were reviewed.
Exposure/Intervention: Sjögren’s syndrome.
Comparison: General people.
Outcomes: The risk of thyroid cancer.
Search Strategy
Databases ProQuest, Cochrane, PubMed, Web of Science, Embase, Scopus and the search engine Google Scholar were searched until may 27, 2025, using the keywords (Sjogren’s Syndrome, Sicca Syndrome, Thyroid Neoplasms, Thyroid Carcinoma, Thyroid Adenoma, Thyroid Cancer) and their MeSH equivalents.The relevant keywords were systematically combined with logical operators, and in the setting of manual search, bibliographies of original studies have been carefully checked. The search strategy in the PubMed database is as follows: (Sjogren's Syndrome OR Sicca Syndrome OR “autoimmune exocrinopathy”) AND (Thyroid Neoplasms OR Thyroid Carcinoma OR Thyroid Adenoma OR Thyroid Cancer OR “papillary thyroid carcinoma,” OR “follicular thyroid cancer”)
Inclusion and Exclusion Criteria
Studies that investigated the association of Sjögren's syndrome with thyroid cancer risk were included in the current study. However, the following studies were excluded from the review process: Case report studies were excluded due to very small sample sizes; Review studies were excluded because if they were not excluded, they would have caused overlap between studies and the effect of a study would have been calculated multiple times; Letter to the editor studies; Replication studies were also excluded, with only one study from each category remaining to avoid overlap; Some studies did not have the necessary data to perform the current meta-analysis and were therefore excluded; Although the corresponding author of the studies whose full text was not available was emailed. We still did not have access to the full text of some studies and the data were incomplete based on the abstract, so they were excluded; Studies that were of poor quality according to the NOS tool and also met one of the other exclusion criteria above; Studies whose abstracts were published as posters at conferences.
Qualitative Assessment
Two researcher evaluated the studies applying the Newcastle Ottawa Scale (NOS) checklist. Each question on this checklist was only allowed to receive one star, with the exception of the comparison question, which may receive two stars. Therefore, the minimum score was zero (lowest quality) and the maximum score was ten (highest quality). The cut-off point in this study was considered to be six [25].
Data Extraction
The following data extracted: researcher, design, age, continent, total number, type of Sjögren’s syndrome, location, year, odds ratio of the association Sjögren’s syndrome with thyroid cancer risk in the entire sample and by gender (along with its upper and lower limit). Two forms were designed and two authors extracted the study data independently. If there was a disagreement, the disagreement was resolved using a consultation and understanding approach, leaving a single and consistent option.
Statistical Analysis
The logarithm of the indices OR, HR, and SIR was used to analyze the collected data, ultimately studies were combined. Because the distribution of the mentioned indicators is not normal, we use their logarithms to normalize them. Raw data is listed. The heterogeneity of the studies was examined using the I2 index (I2=87.8%). Since there was severe heterogeneity in this study, the random effects model was used. Subgroup analysis was used to examine sources of heterogeneity based on study type, continent, patient age group, and duration of involvement. Meta-regression plots were used for additional analysis based on quantitative variables (year of publication and sample size). In addition, funnel plots were used to examine publication bias. Sensitivity analysis was also used to examine which studies were the most influential studies in the current research outcome. Data analysis was done in STATA 14 software (developed by Computing Resource Center in California). The level of significance of the tests was considered to be P<0.05.
Results
After searching databases and Google Scholar, 218 studies were found. Of these, 82 were duplicates and were removed. Then, 136 studies entered the next stage, and after reviewing the information in the abstracts, 25 studies, whose abstract information was incomplete and did not have a full text, were removed. The full text of 111 studies was reviewed, and 33 studies that had not reported the necessary data for data analysis were removed. Consequently, 67 studies were removed based on other exit criteria, and 11 studies remained (Figure-1).
Eleven original articles were reviewed in present research, of which 10 were cohort studies and one was of the Mendelian randomization type, and some of their information is presented in Table-1. According to Figure-2, Sjögren's syndrome increased the risk of thyroid cancer (OR: 2.08, 95%CI: 1.47, 2.94). By comparing the gender of patients with Sjögren's syndrome, we found that women are significantly at risk of thyroid cancer (OR: 1.83, 95%CI: 1.35, 2.48). However, in men, there was no statistically significant association between Sjögren's syndrome and the risk of thyroid cancer (OR: 1.49, 95%CI: 0.95, 2.34).
Due to the heterogeneity between studies, a random effects model was used (I2=87.8%). Because when the I2 is greater than 75%, a random effects model is used. The risk of thyroid cancer in patients with Sjögren's syndrome increased in the ages of 40 to 49 years (OR: 1.43, 95%CI: 1.23, 1.67), 50 to 59 years (OR: 4.65, 95%CI: 1.87, 11.58), and 60 to 69 years (OR: 1.34, 95%CI: 1.08, 1.66), showing that patients in their fifth decade of life are more at risk of thyroid cancer (Figure-3). Figure-4 indicated that the relationship between Sjögren's syndrome and the risk of thyroid cancer in the Mendelian randomization study was not statistically significant (OR: 0.88, 95%CI: 0.70, 1.10). However, in cohort studies, the risk of thyroid cancer was significantly increased due to Sjögren's disease. (OR: 2.35, 95%CI: 1.61, 3.41). In examining the continents, it was found that European patients with Sjögren's syndrome (OR: 3.26, 95%CI: 1.24, 8.56) are more at risk of thyroid cancer than Asian patients (OR:1.87, 95%CI: 1.27, 2.74, Figure-5). Patients with Sjögren's syndrome who had a follow-up period of less than or equal to 5 years (OR: 1.68, 95%CI: 1.10, 2.54) and patients with a follow-up period of more than 5 years (OR:5.77, 95%CI: 1.97, 16.97) were at risk of thyroid cancer (Figure-6). Figure-7 demonstrated that patients with primary Sjögren's syndrome were more at risk of thyroid cancer than ordinary people (OR: 2.37, 95%CI: 1.44, 3.90). Since other studies did not specify the type of Sjögren's, no analysis was performed based on patients with secondary Sjögren's syndrome. In the additional analyses section, meta-regression revealed that there was no statistically significant relationship between “the relationship between Sjögren's syndrome and the risk of thyroid cancer” and the year of publication of the studies (P=0.511). Similarly, the relationship between “the relationship between Sjögren's syndrome and the risk of thyroid cancer” and the sample size of the studies was also not statistically significant (P=0.374, Figures-8 and -9). In Figure-10, the sensitivity analysis showed that the study by Zhou et al. in 2022 and the study by Jia et al. in 2023 were the most influential studies in the final result of the current research. Much so that the result of the current research changed to (OR: 0.52, 95%CI: 0.22, 0.81) when the Zhou study was removed. Moreover, the current research's conclusion was also changed to (OR: 0.85, 95%CI: 0.48, 1.23) when the Jia study was removed. The publication bias graph was significant (P=0.026) and showed that studies that did not report a significant association between Sjögren's syndrome and thyroid cancer risk had little chance of being published (Figure-11).
Discussion
Sjögren’s syndrome, especially primary Sjögren’s syndrome, increased the risk of thyroid cancer. In men, there was no significant association between Sjögren’s syndrome and the risk of thyroid cancer. However, Sjögren’s syndrome in women increases their risk of thyroid cancer.
Although in the fourth, fifth, and sixth decades of life, having Sjögren’s syndrome increases the risk of thyroid cancer. However, it was not the case that as the age of patients with Sjögren’s syndrome increased, the risk of thyroid cancer also increased, and the fifth decade of life was the most dangerous decade for the onset of thyroid cancer. Of course, since the distribution of studies among different age groups was different, this may have caused a direct relationship between the age of patients and the increased risk of thyroid cancer not being seen. In addition, patients who had been involved for more than 5 years were significantly more at risk of thyroid cancer than patients who had been involved for less than or equal to 5 years. That is, the longer the involvement, the greater the likelihood of developing thyroid cancer. Furthermore, the risk of thyroid cancer in patients with Sjögren’s syndrome in Europe was higher than in Asian patients.
Previous studies have shown that postmenopausal women are at increased risk of thyroid cancer due to disruption in sex hormone levels. The results showed that the decline in sex hormones such as FSH and TH in patients increased the risk of cancer compared to normal individuals. This was while some other studies had challenged these findings. Therefore, it can be said that there is no definitive evidence regarding the effect of hormones on the incidence of thyroid cancer and further studies are needed in the future [31, 32].
The results of the present study showed that the frequency of thyroid cancer was higher in Europe compared to Asia. This difference could be influenced by various factors. Previous studies have shown that iodine deficiency was higher in Europe due to diet. On the other hand, autoimmune diseases such as Sjögren's syndrome are associated with HLA. Therefore, it is possible that the frequency of a particular HLA is higher in a region, thus increasing the risk of autoimmune diseases [33, 34].
In a systematic review study by Kaur and colleagues (2022), the results showed that patients with Sjögren’s syndrome are at serious risk of thyroid disorders, especially autoimmune thyroiditis [35].
In a meta-analysis conducted by Sun and colleagues (2019), to investigate the risk of thyroid disease in patients with Sjögren’s syndrome, the results showed that the risk of developing thyroid disease in patients with Sjögren’s syndrome was significantly higher compared to the control group (OR: 3.29, 95% CI: 2.08, 5.21) [36]. The results of these studies were in line with the results of the current study and showed that having Sjögren’s syndrome is a serious risk factor for the development of thyroid disorders.
Liang and colleagues (2014) conducted a meta-analysis study and they aimed to investigate the relationship between primary Sjögren’s syndrome and the risk of malignancy. Ultimately, the results showed that the risk of thyroid cancer in patients with primary Sjögren’s syndrome was significantly higher compared to the general population (RR: 2.58, 95% CI: 1.14, 4.03) [37]. In another meta-analysis conducted by Zhong and colleagues (2022), intending to investigate the relationship between primary Sjögren’s syndrome and the risk of malignancy, primary Sjögren’s syndrome significantly increased the risk of solid tumors (SIR: 1.39, 95%CI: 0.90, 2.13) including thyroid cancer (SIR: 2.05, 95%CI: 1.20, 3.48) [38]. In another study, Batista and colleagues (2023) using the meta-analysis method indicated that the risk of thyroid cancer in patients with autoimmune rheumatic diseases was higher than in the general population (SIR: 1.76, 95% CI: 1.31, 2.37). Moreover, the risk of thyroid cancer in patients with Sjögren’s syndrome was higher compared to the general population (SIR: 2.97, 95% CI: 2.31, 3.82) [39]. The results of these studies were in line with the results of the current study. The above studies showed that Sjögren’s syndrome increases the risk of thyroid cancer. This association is evident because the thyroid is one of the organs involved in the problems of Sjögren's syndrome.
Previous studies concentrated on the possible connection between Sjögren's syndrome and a few types of cancer. In one study, Zadori and colleagues (2021) investigated the relationship between autoimmune disorders and the onset of stomach cancer using the meta-analysis method. Zadori’s investigation indicated that there was no significant relationship between stomach cancer and Sjögren’s syndrome (SIR: 1.19, 95%CI: 0.85, 1.66)) [40]. In a meta-analysis conducted by Deng and colleagues (2022) to investigate the risk, incidence, and mortality of breast cancer in primary Sjögren’s syndrome, primary Sjögren’s syndrome had no relationship with the risk of breast cancer (SIR: 0.92, 95%CI: 0.66, 1.29) [41]. These studies' findings differed from the findings of the current investigation, and Sjögren’s syndrome did not raise the risk of stomach and breast cancers. Of course, the type of disease, the age of patients, and the duration of the disease are among the important factors that if examined separately, can change the result of the study. Conversely, in the following study, researchers found that primary Sjögren’s syndrome increased the risk of non-Hodgkin’s lymphoma. In a meta-analysis conducted by Ansari and colleagues (2024) to investigate the relationship between primary Sjögren’s syndrome and non-Hodgkin’s lymphoma, the findings showed that there is a significant relationship between primary Sjögren’s syndrome and the risk of non-Hodgkin’s lymphoma (SIR: 8.78, 95% CI: 5.51, 13.99) [42].
Given that this study showed that the incidence of thyroid cancer was higher in women, it is better for women to be screened regularly after menopause so that patients can be managed with timely diagnosis.
The incidence of thyroid and breast cancer in patients can vary. This difference can be due to the physiology of each of the two organs. On the other hand, the biological processes of the two organs, the thyroid and the breast, can play an important role in the occurrence of cancer [43, 44].
Conclusions
Sjögren’s syndrome increased the risk of thyroid cancer, and female gender, the fifth decade of life, European race, and involvement duration of more than 5 years were among the factors that increased this probability. In this study, the relationship between Sjögren’s syndrome and the risk of thyroid cancer was examined based on different subgroups. Therefore, it is recommended that researchers in future studies separately investigate the relationship between primary and secondary Sjögren’s syndrome with the risk of thyroid cancer. Moreover, in two groups of patients with primary and secondary Sjögren’s syndrome, evaluate the relationship between each of the subgroups of age, gender, duration of involvement, and ethnicity with thyroid cancer independently.
Acknowledgment
We wish to thank all our colleagues in the Isfahan University of Medical Science.
Conflict of Interest
The authors declare that they have no conflict of interest.
|
GMJ Copyright© 2025, Galen Medical Journal. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/) Email:gmj@salviapub.com |
-web-resources/image/QR_Code193401064-889500093.png)
|
Correspondence to: Mansour Salesi, Isfahan University of Medical Science, Isfahan, Iran. Telephone Number: 09131210325 Email Address: salesi@med.mui.ac.ir |
|
GMJ.2025;14:e3753 |
www.salviapub.com
|
Salesi M |
Sjögren Syndrome and Thyroid Cancer Risk |
|
2 |
GMJ.2025;14:e3753 www.gmj.ir |
|
Sjögren Syndrome and Thyroid Cancer Risk |
Salesi M |
|
GMJ.2025;14:e3753 www.gmj.ir |
3 |
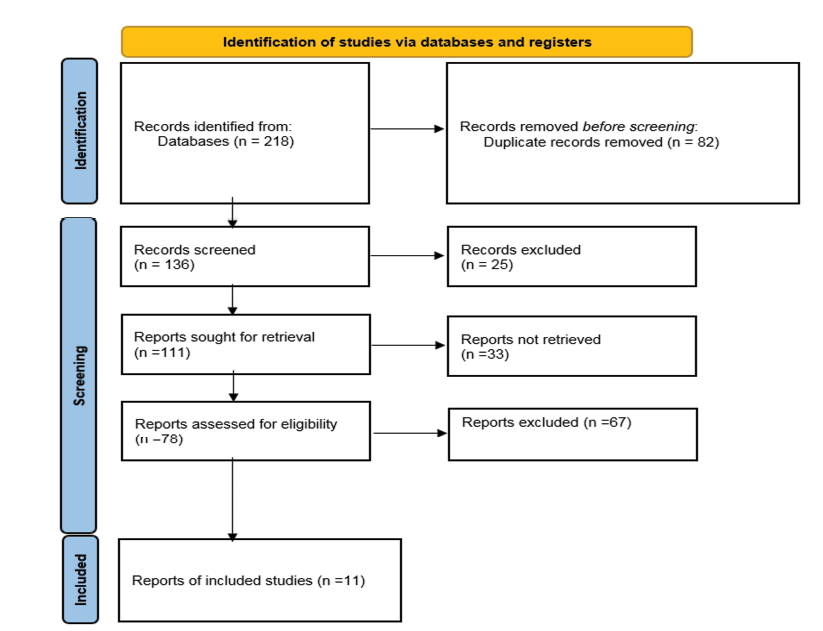
Figure 1. Chart of PRISMA
|
Salesi M |
Sjögren Syndrome and Thyroid Cancer Risk |
|
4 |
GMJ.2025;14:e3753 www.gmj.ir |
|
Sjögren Syndrome and Thyroid Cancer Risk |
Salesi M |
|
GMJ.2025;14:e3753 www.gmj.ir |
5 |
Table 1. Characteristics of Articles
|
References |
Index |
Continent |
Place |
Type of Sjogren’s Syndrome |
Type of study |
Total number |
Mean age |
Duration of study |
Follow-up (Year) |
Risk of thyroid cancer |
Low limit |
Up limit |
|
Park SK, 2024[20] |
HR |
Asia |
South Korea |
NR |
Cohort |
17752 |
60-69 |
2002 to 2019 |
9.49 |
1.48 |
0.91 |
2.39 |
|
Yang TH, 2023[22] |
OR |
Asia |
Taiwan |
NR |
Cohort |
194650 |
40-49 |
between January 2012, and December 2019 |
NR |
1.43 |
1.23 |
1.67 |
|
Jia Y, 2023[21] |
OR |
Europe |
Europe |
NR |
Mendelian randomization |
1080 |
NR |
NR |
NR |
0.88 |
0.7 |
1.1 |
|
Zhou Z, 2022[23] |
SIR |
Asia |
China |
NR |
Cohort |
1329 |
50-59 |
between January 2006 and April 2015 |
6.65 |
8.41 |
4.34 |
14.68 |
|
Isık OO, 2022[26] |
SIR |
Europe |
Turkey |
Primary |
Cohort |
151 |
50-59 |
between 2004 and 2019 |
10.5 |
23.07 |
4.8 |
67.4 |
|
Goulabchand R, 2021[27] |
HR |
Europe |
France |
Primary |
Cohort |
278204 |
60-69 |
from 2011 to 2018 |
3.96 |
1.73 |
1.07 |
2.79 |
|
Kang J, 2020[28] |
SIR |
Asia |
South Korea |
Primary |
Cohort |
5482661 |
60-69 |
between January 2012 and December 2014 |
NR |
1.19 |
0.87 |
1.52 |
|
Ahn JK, 2020[29] |
SIR |
Asia |
South Korea |
Primary |
Cohort |
NR |
50-59 |
between 2007 and 2017 |
3.1 |
1.23 |
0.88 |
1.68 |
|
Brito-Zeron P, 2017[19] |
SIR |
Europe |
Spain |
Primary |
Cohort |
1300 |
50-59 |
2005-2016 |
7.58 |
5.05 |
1.89 |
13.45 |
|
Weng MY, 2012[18] |
SIR |
Asia |
Taiwan |
Primary |
Cohort |
7852 |
50-59 |
from 2000 to 2008 |
3.5 |
2.56 |
1.4 |
4.3 |
|
Theander E, 2006[30] |
SIR |
Europe |
Sweden |
Primary |
Cohort |
194 |
50-59 |
from 1984 until 31 December 2002 |
8 |
6.86 |
0.17 |
38.21 |
|
Salesi M |
Sjögren Syndrome and Thyroid Cancer Risk |
|
6 |
GMJ.2025;14:e3753 www.gmj.ir |
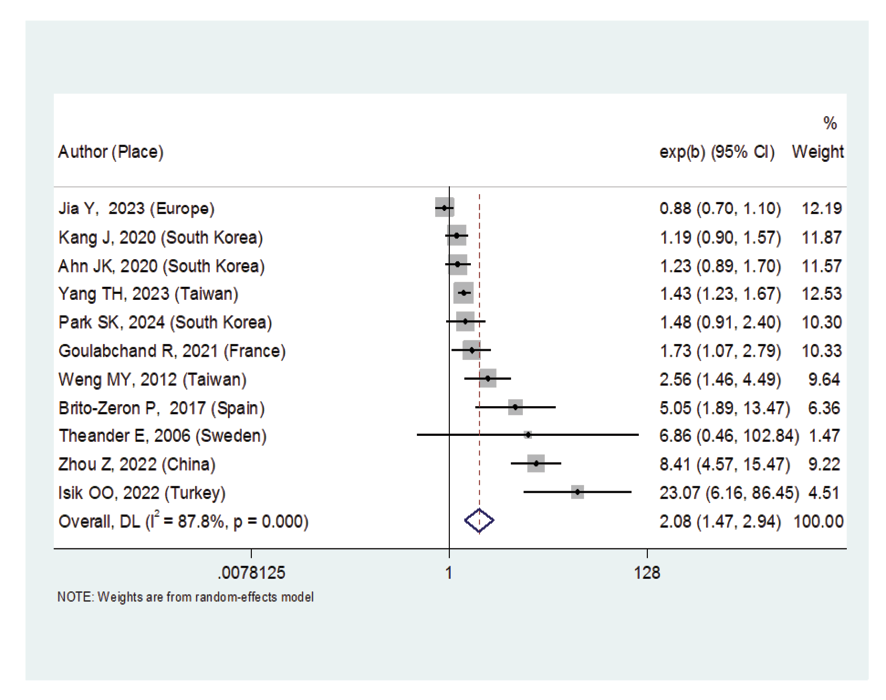
Figure 2. Forest plot related to the association between Sjogren's syndrome and thyroid cancer risk
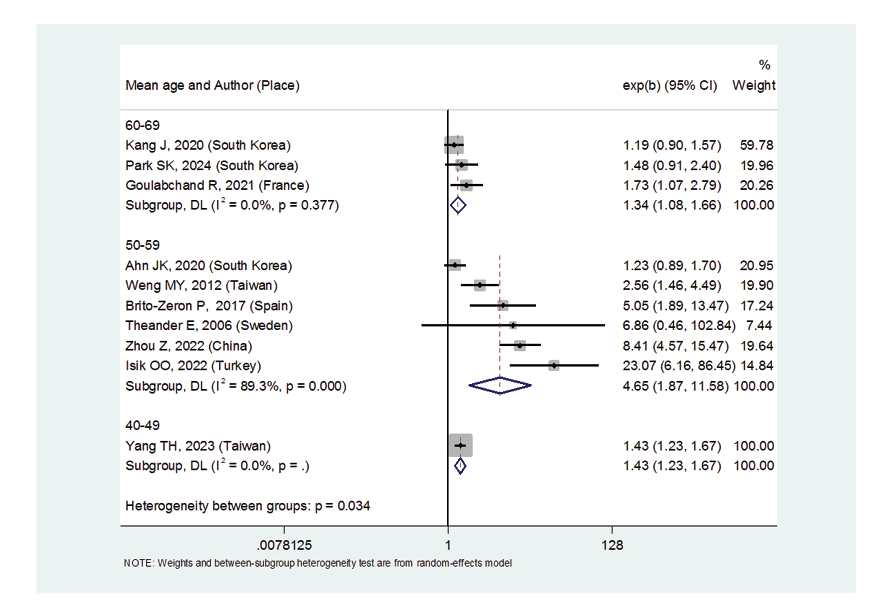
Figure 3. Forest plot related to the association between Sjogren’s syndrome and thyroid cancer risk by mean age
|
Sjögren Syndrome and Thyroid Cancer Risk |
Salesi M |
|
GMJ.2025;14:e3753 www.gmj.ir |
7 |
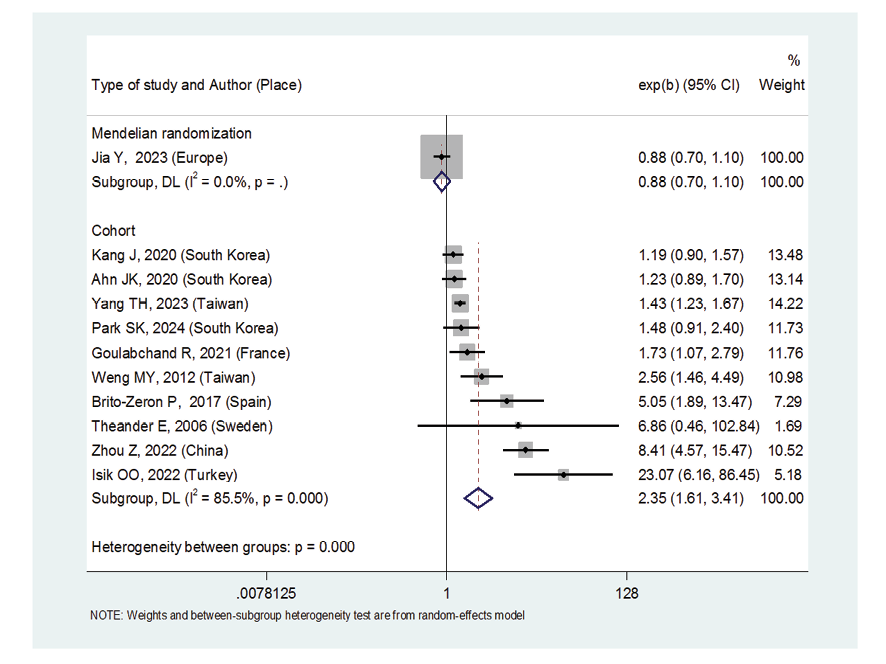
Figure 4. Forest plot related to the association between Sjogren's syndrome and thyroid cancer risk by type of study
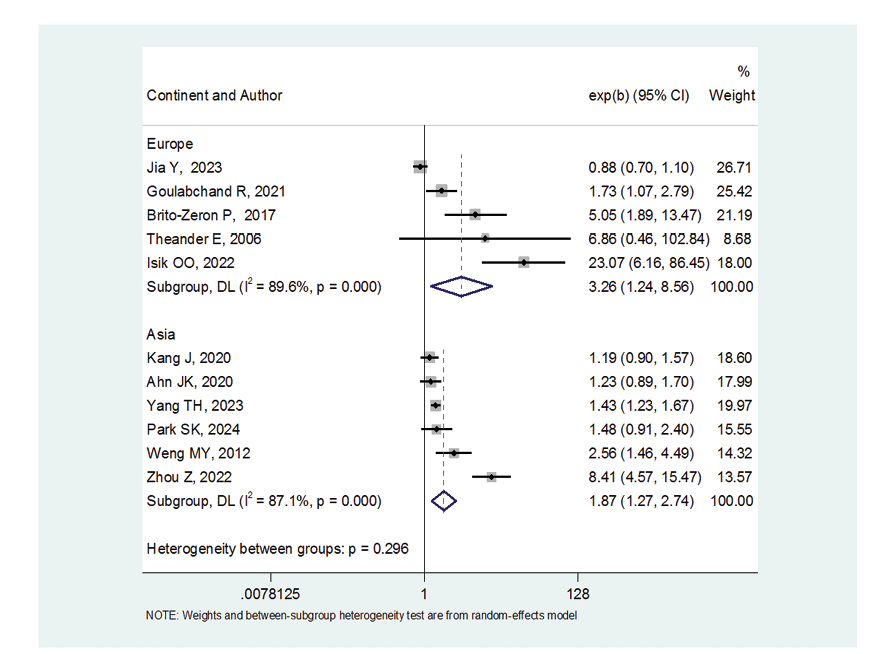
Figure 5. Forest plot related to the association between Sjogren's syndrome and thyroid cancer risk by continent
|
Salesi M |
Sjögren Syndrome and Thyroid Cancer Risk |
|
8 |
GMJ.2025;14:e3753 www.gmj.ir |
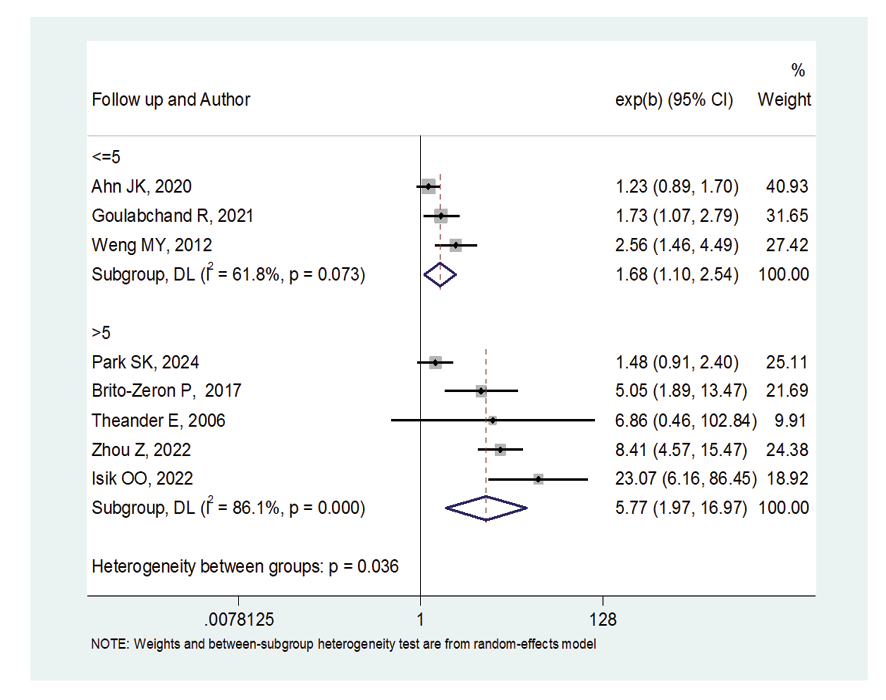
Figure 6. Forest plot related to the association between Sjogren's syndrome and thyroid cancer risk by follow up
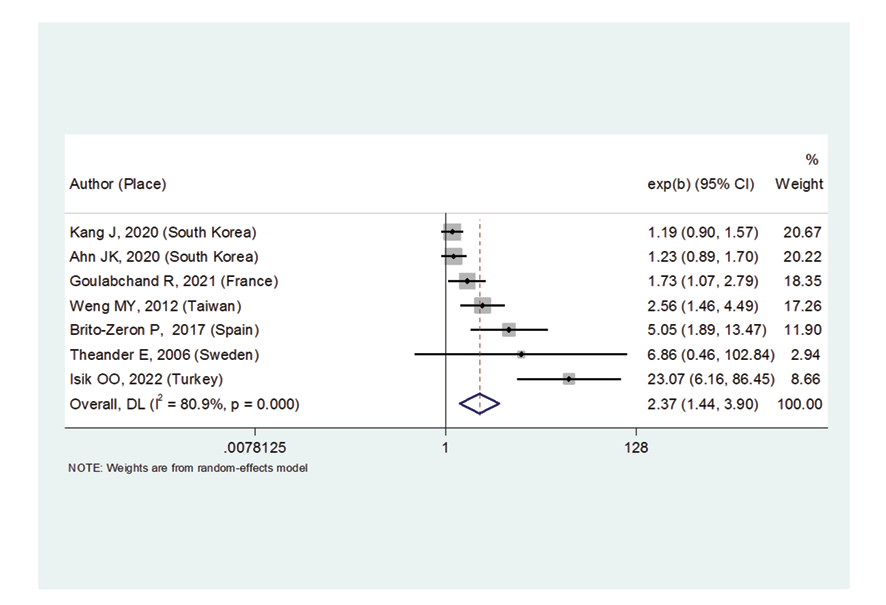
Figure 7. Forest plot related to the association between primary Sjogren's syndrome and thyroid cancer risk
|
Sjögren Syndrome and Thyroid Cancer Risk |
Salesi M |
|
8 |
GMJ.2025;14:e3753 www.gmj.ir |
|
GMJ.2025;14:e3753 www.gmj.ir |
9 |
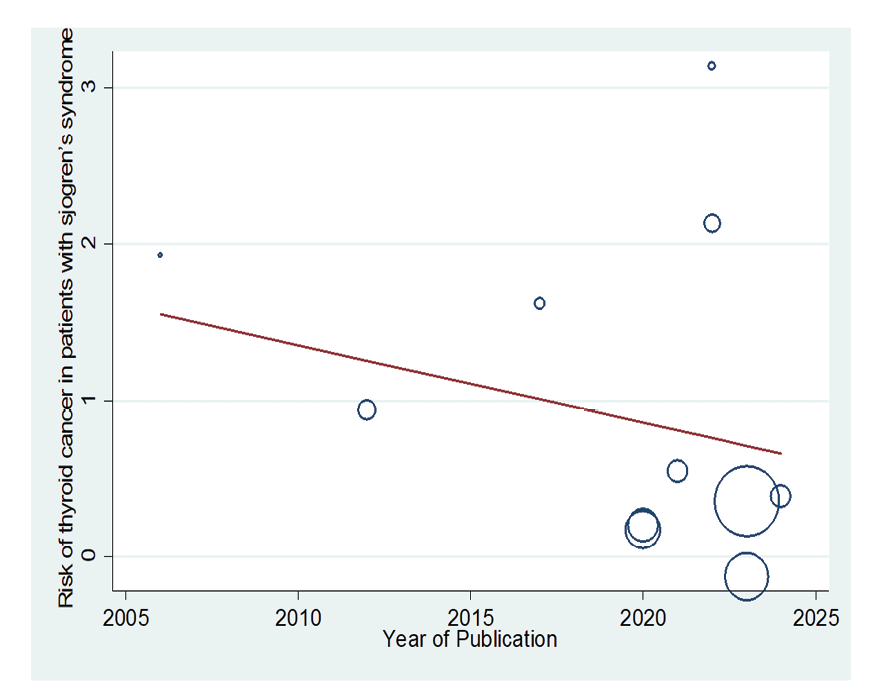
Figure 8. Meta-regression plot of the relationship between “association between Sjogren's syndrome and thyroid cancer risk” year of publication
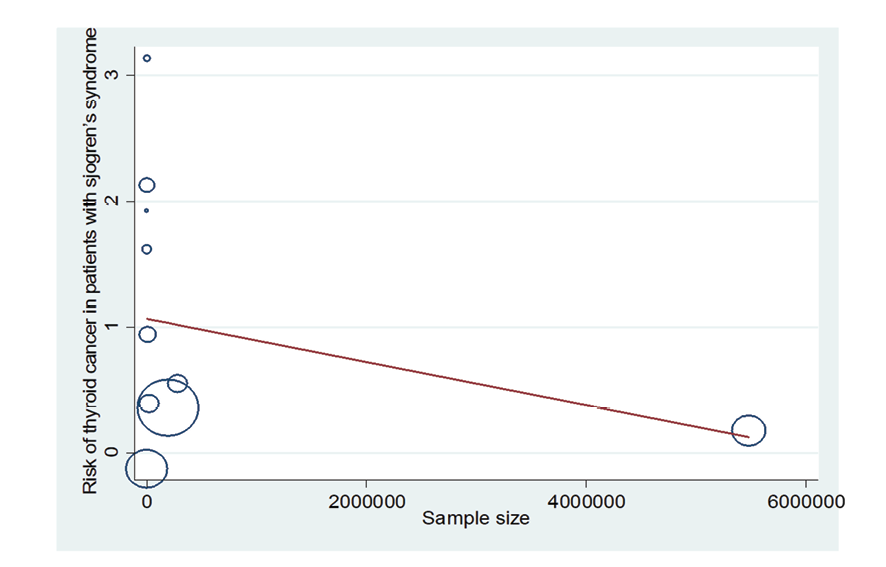
Figure 9. Meta-regression plot of the relationship between “association between Sjogren's syndrome and thyroid cancer risk” and sample size
|
Salesi M |
Sjögren Syndrome and Thyroid Cancer Risk |
|
10 |
GMJ.2025;14:e3753 www.gmj.ir |
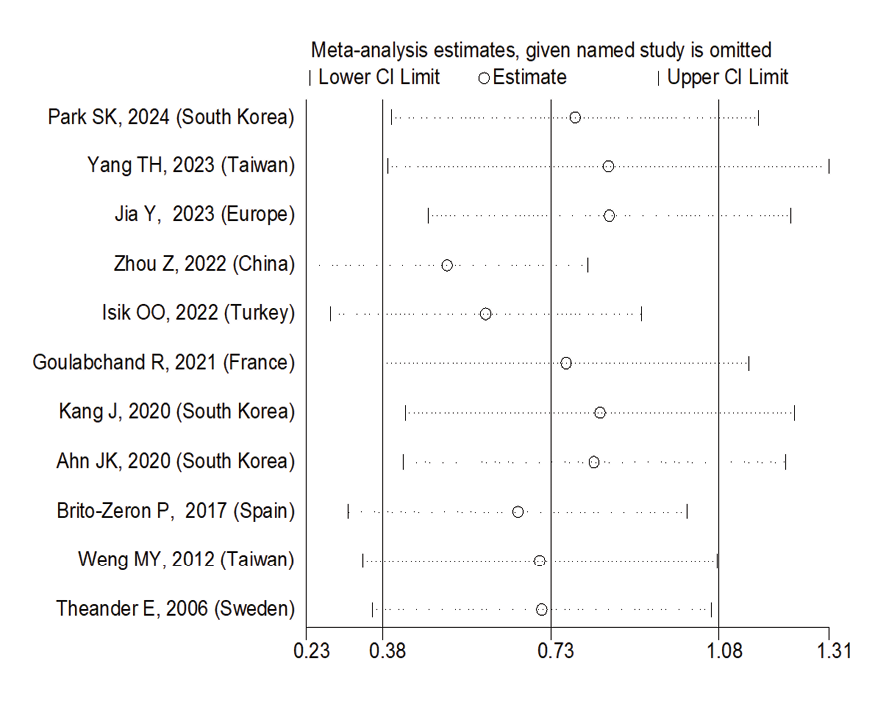
Figure 10. Sensitive analysis
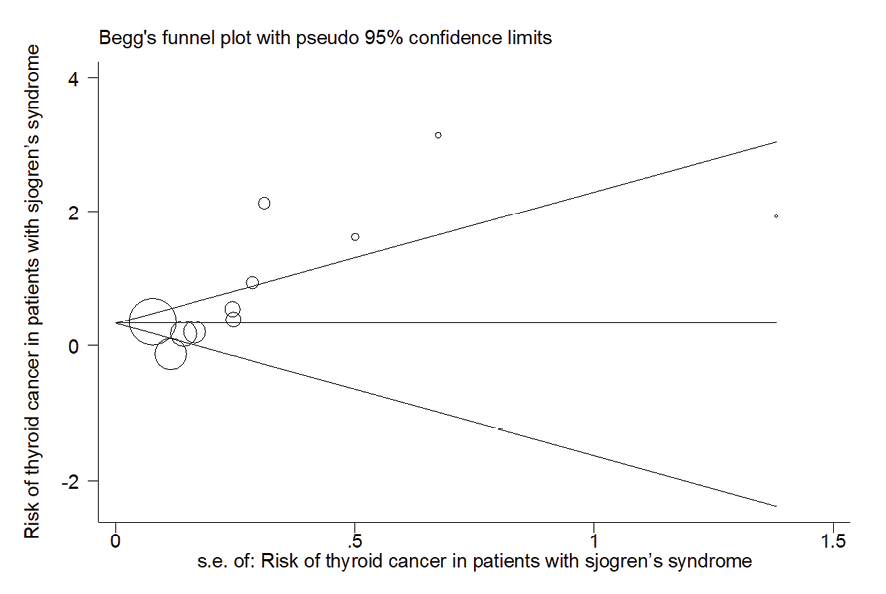
Figure 11. Publication bias diagram
|
Sjögren Syndrome and Thyroid Cancer Risk |
Salesi M |
|
8 |
GMJ.2025;14:e3753 www.gmj.ir |
|
GMJ.2025;14:e3753 www.gmj.ir |
11 |
|
Salesi M |
Sjögren Syndrome and Thyroid Cancer Risk |
|
12 |
GMJ.2025;14:e3753 www.gmj.ir |
|
References |
|
Salesi M |
Sjögren Syndrome and Thyroid Cancer Risk |
|
8 |
GMJ.2025;14:e3753 www.gmj.ir |
|
GMJ.2025;14:e3753 www.gmj.ir |
13 |
|
Salesi M |
Sjögren Syndrome and Thyroid Cancer Risk |
|
14 |
GMJ.2025;14:e3753 www.gmj.ir |