

Received 2025-01-02
Revised 2025-02-05
Accepted 2025-03-16
Radiographic Evaluation of Implant Stability and Osseointegration in Adult Orthodontic Patients
Sajjad Rostamzadeh 1, Mohammad Ghasemirad 2, Mohammad Gerayeli 3, Mina Abasi 4,
Mohsen Pouresmaeliyan Roumani 5, Shabnam Ganjehzadeh 3, Amirmohammad Moharrami 6
1 Department of Orthodontics, Faculty of Dentistry, Tabriz University of Medical Sciences, Tabriz, Iran
2 Department of Periodontics, Faculty of Dentistry, Rafsanjan University of Medical Sciences, Rafsanjan, Iran
3 Dental Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
4 School of Dentistry, Tehran University of Medical Sciences, Tehran, Iran
5 Private Dental Clinic, Shahrebabak, Kerman,Iran
6 Department Of Orthodontics, Faculty of Dentistry, Tehran University of Medical Sciences, Tehran, Iran
|
Abstract Radiographic evaluation is essential for assessing implant stability and osseointegration in adult orthodontic patients. The success of temporary anchorage devices (TADs) and mini-implants depends on primary stability, achieved through mechanical engagement, and secondary stability, influenced by bone remodeling. While traditional clinical methods, such as mobility testing, provide subjective assessments, radiographic imaging offers objective insights into bone-implant interactions. Periapical and panoramic radiographs are commonly used but are limited by their two-dimensional (2D) nature. Cone beam computed tomography (CBCT) has emerged as the gold standard, providing three-dimensional (3D) visualization of cortical bone thickness, marginal bone loss, and peri-implant adaptations. However, challenges such as image artifacts, radiation exposure, and observer variability persist. Implant stability is influenced by factors like bone density, cortical thickness, insertion torque, and patient-specific variables, including systemic conditions, genetic predisposition, and lifestyle habits. Emerging techniques such as resonance frequency analysis (RFA) complement radiographic findings by providing quantitative stability assessments. Additionally, artificial intelligence (AI)-driven radiographic analysis is improving diagnostic accuracy, automating bone density evaluation, and predicting implant success. Future advancements in low-dose CBCT protocols, AI-assisted diagnostics, and digital treatment planning aim to optimize implant placement and long-term stability assessment. By integrating multimodal imaging approaches with biomechanical and AI-driven predictive modeling, clinicians can enhance treatment planning, reduce implant failure rates, and improve orthodontic outcomes. This review underscores the importance of advanced imaging techniques in implant stability assessment and highlights the need for continued research in AI-driven diagnostics and minimally invasive evaluation methods. [GMJ.2025;14:e3799] DOI:3799 Keywords: Implant Stability; Osseointegration; CBCT; Orthodontic Mini-implants; Temporary Anchorage Devices (TADs); Artificial Intelligence; Peri-implant Bone Loss |
|
GMJ Copyright© 2025, Galen Medical Journal. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/) Email:gmj@salviapub.com |

|
Correspondence to: Amirmohammad Moharrami, Department Of Orthodontics, Faculty of Dentistry, Tehran University of Medical Sciences, Tehran, Iran. Telephone Number: 041 3335 5965 Email Address: amir.orthodent@gmail.com |
|
GMJ.2025;14:e3799 |
www.salviapub.com
|
Rostamzadeh S, et al. |
Radiographic Evaluation of Implant Stability and Osseointegration |
|
2 |
GMJ.2025;14:e3799 www.gmj.ir |
Introduction
Implant stability and osseointegration are fundamental determinants of the success of dental implants in orthodontic applications [1]. The increasing use of temporary anchorage devices (TADs) and orthodontic mini-implants has revolutionized treatment modalities, providing predictable anchorage with minimal patient compliance [2].
However, the long-term success of these implants is contingent upon their ability to achieve and maintain stability within the bone [3]. A key factor influencing implant longevity is the process of osseointegration, which is characterized by the direct structural and functional connection between bone and implant surface [4]. Given the dynamic biomechanical forces present in orthodontic treatment, the assessment of implant stability is particularly critical [5].
Radiographic evaluation plays a pivotal role in assessing implant stability and osseointegration, offering objective insights into bone-implant interactions [6]. Traditional clinical methods, such as tactile assessment and mobility testing, provide only subjective and qualitative data, often failing to detect early complications or subtle peri-implant changes [7].
Advanced imaging techniques, including periapical and panoramic radiographs, as well as cone beam computed tomography (CBCT), have significantly improved the ability to assess marginal bone levels, cortical engagement, and bone density changes over time [1]. While periapical radiographs offer high-resolution imaging for localized bone evaluation, they are limited by their two-dimensional (2D) nature, which may obscure buccal and lingual bone changes [5].
Similarly, panoramic radiographs, though useful for broad anatomical assessment, have been criticized for their inherent distortion and lower accuracy in detecting early peri-implant bone loss [8].
Recent studies emphasize the role of CBCT in providing three-dimensional (3D) visualization, allowing for a more precise assessment of implant positioning and peri-implant bone adaptations [2]. For instance, Cui et al. [9] provided valuable insights into the variations in crestal soft tissue thickness using this technique ; their findings revealed only a weak correlation between soft and hard tissue measurement. However, the study's retrospective design and limited anterior site sample size may restrict its clinical applicability [9]. Additionally, image artifacts from metallic restorations may affect CBCT accuracy, requiring further refinement of metal artifact reduction algorithms to ensure reliable diagnostics [7, 10]. While this technique remains the most comprehensive imaging modality for implant evaluation, its use should be strategically balanced against radiation exposure risks, particularly in routine monitoring [5].
The importance of radiographic evaluation extends beyond initial implant placement, playing a crucial role in predicting implant success [11].
Bone remodeling, a continuous process of resorption and deposition, can significantly influence implant stability over time [4] Factors such as implant loading conditions, bone quality, and patient-specific variables contribute to variations in osseointegration, necessitating longitudinal radiographic monitoring [5].
Early detection of bone loss or peri-implant radiolucency allows for timely intervention, preventing implant failure [1]. Furthermore, the integration of artificial intelligence (AI) in radiographic assessment has opened new avenues for automated analysis, improving diagnostic precision and reducing observer variability [6].
Several radiographic modalities are currently employed to evaluate implant stability, each offering distinct advantages and limitations. Periapical radiographs, widely used for assessing marginal bone levels, provide high-resolution imaging but are limited by their two-dimensional (2D) nature[3, 5]. Panoramic radiographs offer a broader field of view, facilitating overall treatment planning but with reduced image sharpness [2]. CBCT, as the gold standard for 3D imaging, enables comprehensive assessment of bone volume, cortical engagement, and implant angulation[11]. This review aims to provide a comprehensive analysis of radiographic techniques used for evaluating implant stability and osseointegration in adult orthodontic patients.
Biomechanics of Implant Stability in Orthodontics
Implant stability is a fundamental requirement for the success of orthodontic implants, including TADs and mini-implants. Stability ensures the implant’s ability to resist micromovements under functional loads, which is critical for achieving predictable orthodontic [12]. Implant stability is classified into two distinct phases: primary stability, which is mechanically achieved at the time of insertion, and secondary stability, which results from biological remodeling and osseointegration over [13]. A clear understanding of the biomechanical principles governing implant stability is essential for optimizing clinical protocols, improving implant success rates, and minimizing failure risks in orthodontic patients [12].
Primary vs. Secondary Stability
Primary stability is the initial mechanical engagement of the implant with the surrounding bone, primarily dependent on bone quality, implant geometry, and insertion technique [13]. In orthodontic applications, mini-implants and TADs rely heavily on cortical bone engagement to achieve sufficient primary stability [14, 15]. Studies have shown that high insertion torque (>10 Ncm) is associated with improved primary stability, as it ensures adequate bone-implant contact and reduces micromovements that could disrupt osseointegration[16, 17].
However, excessive torque may cause microdamage to the bone, potentially leading to implant failure [18].
Secondary stability develops over time as a result of biological processes, including bone remodeling and osseointegration [19]. Unlike endosseous dental implants, which rely heavily on osseointegration for long-term stability, orthodontic mini-implants often function through mechanical retention without complete osseointegration. Nevertheless, peri-implant bone adaptation and remodeling influence implant longevity. A study by Monje et al.[12] demonstrated that increased cortical bone thickness enhances secondary stability by providing greater resistance to micromovements, thereby reducing the risk of early implant failure. This highlights the importance of patient-specific bone characteristics in treatment planning [12].
The transition from primary to secondary stability is a critical period where implant micromovements must be minimized to prevent fibrous encapsulation, which can lead to implant loosening [20]. If excessive mobility exceeds 50-150 µm, the formation of a fibrous interface instead of direct bone contact may occur, compromising implant retention [21]. This underscores the significance of controlled loading conditions during the early phases of implantation [22].
Factors Influencing Implant Stability
Table-1 demonstrated common factors affecting implant stability. Implant stability is fundamentally influenced by bone quality, design parameters, and biomechanical loading [23]. Denser bone with greater cortical thickness provides superior mechanical interlocking, making it a critical factor for both conventional implants and TADs [24]. Regions such as the adult mandible typically offer enhanced primary stability due to their dense and thick cortical structure. In contrast, sites with low-density trabecular bone or thin corticeslike the posterior maxilla or in adolescent patients often yield lower insertion torque and higher failure rates[23, 25, 26].
Lee et al [25]. found that cancellous bone density had a greater effect on miniscrew success than cortical thickness alone. Also, Truong et al [27]. similarly emphasized bone mineral density as the key determinant in TAD migration under load. Additionally, implant design contributes substantially for TADs, longer screws (≥8 mm) and diameters of 1.5–1.6 mm optimize retention while minimizing cortical trauma [27]. Tapered screw geometries yield higher primary stability than cylindrical designs [28], while thread design and insertion technique (self-drilling vs. pre-drilling) are less influential [27].
In conventional implants, tapered and hybrid geometries outperform parallel-walled implants in soft bone [29, 30]. However, surface roughness primarily supports osseointegration, not immediate mechanical stability [29]. Loading protocols and biomechanical factors further influence outcomes. Immediate loading, placing the prosthetic shortly after implant placement, has shown comparable survival rates to delayed loading when primary stability is sufficient (insertion torque ≥30–35 Ncm or ISQ ≥60) [31]. Orthodontic mini-screws, in contrast, are usually loaded immediately and require careful attention to force magnitude and duration [32]. Excessive insertion torque or omitting pilot drilling can cause cortical microdamage, reducing initial retention [33]. Truong et al. reported that bone trauma during insertion compromises early stability , though pilot drilling with smaller diameters can mitigate this risk [27]. Bicortical anchorage techniques improve resistance to displacement over time compared to monocortical anchorage [34], but even well-placed screws exhibit some degree of “creep” under sustained load [35]. Long-term success also depends on biological integration. Conventional implants experience an initial dip in stability as bone remodels, then recover through secondary stability supported by surface roughness [36].
Finally, patient-specific factors such as age, systemic health, oral hygiene, and anatomical site must be considered. Stability is generally lower in younger patients with immature bone [37], and conditions like diabetes and osteoporosis impair osseointegration [38]. Inflammatory conditions, poor hygiene, and unfavorable soft tissue can also compromise stability[24]. Customized planning using 3D imaging is therefore essential to identify optimal insertion sites, avoid anatomical risks, and maximize primary retention [39].
Radiographic Techniques for Evaluating Implant Stability
Accurate assessment of implant stability is crucial for ensuring long-term success in implant dentistry [40]. Radiographic techniques provide essential information on peri-implant bone levels, implant positioning, and potential complications. Various imaging modalities differ in their resolution, depth of information, and clinical application [41]. Selecting the appropriate technique depends on the stage of treatment, the complexity of the case, and the required diagnostic precision [42]. Table-2 shows the comparation of current and future radiographic techniques for Implant Stability.
Periapical Radiography
Periapical Radiography is widely used for early implant evaluation due to its high resolution and ability to detect marginal bone loss. It provides detailed imaging of the implant and surrounding bone, making it suitable for monitoring crestal bone changes [43]. However, its two-dimensional (2D) nature limits its ability to assess buccal and lingual bone defects. Despite these limitations, periapical radiographs remain a primary tool in routine follow-ups, particularly in cases where vertical bone loss is of concern [44].
Panoramic Radiography
Panoramic Radiography offers a broader field of view, making it useful for evaluating multiple implants, adjacent anatomical structures, and overall bone levels [45]. It provides essential preoperative information for implant planning but lacks the fine detail required to assess early peri-implant changes accurately. The inherent distortion and magnification errors associated with panoramic images can affect precision; thus, they are often complemented by other imaging methods for more detailed assessments [44].
CBCT
CBCT is now widely used in implant dentistry to overcome many limitations of planar radiographs. This technique generates true 3D volumetric images of the jaws, allowing visualization of the bone implant interface in all dimensions [46]. Unlike 2D films, CBCT can measure bone thickness and volume around implants, detect buccal or lingual dehiscences, and identify peri-implant defects that lie outside the narrow field of a periapical film [47]. Its superiority over conventional 2D radiographs lies in its ability to detect buccal and lingual cortical bone changes, which are critical in assessing implant stability [48]. However, the higher radiation dose compared to traditional radiographs necessitates judicious use, particularly in follow-up assessments [46].
Clinical Application and Challenges of Radiographic Techniques
Radiographic imaging is a cornerstone of clinical management for both temporary orthodontic implants (TADs/mini-screws) and conventional dental implants. Clinicians routinely use intraoral and panoramic radiographs to verify implant position and to monitor marginal bone levels over time, while CBCT) provides detailed three-dimensional assessment of bone anatomy and implant orientation [43, 49]. In orthodontic cases, CBCT also aids in planning mini-implant placement by identifying interradicular bone volume and root positions; studies show that CBCT-guided placement yields higher accuracy and fewer root perforations than two-dimensional radiographs alone [50, 51]. After insertion, radiographic follow-up is used to detect signs of osseointegration or instability. Conventional implants are monitored for crestal bone loss (an indicator of peri-implant health) and any radiolucency at the implant interface [51].
Despite these applications, radiographic evaluation faces several important challenges:
• Technical imaging limitations: Two-dimensional images suffer from overlap and distortion. Panoramic and periapical films cannot depict the bucco-lingual dimension and may compress interradicular spaces, making depth assessment difficult [50]. While CBCT overcomes many projection issues by providing multi-planar 3D views, it introduces other limitations [52]. CBCT resolution, although high, still does not resolve the microscopic bone implant interface even sophisticated micro-CT and synchrotron imaging studies indicate that the first few millimeters of bone contact cannot be distinguished on clinical radiographs [53] . Metal artifacts are a further problem; orthodontic brackets, wires or implant fixtures can create streaks and noise that obscure peri-implant bone details [49]. Moreover, there is no universal standard for image acquisition and calibration. Radiographic gray values (in CBCT or digital X-rays) are not directly comparable across machines or settings, complicating attempts to quantify bone density or detect subtle changes [49]. Low-dose CBCT protocols have been developed to reduce radiation, but lowering dose often comes at the expense of image quality and contrast, potentially impairing the detection of early bone changes [49, 54].
• Diagnostic interpretation variability: Reading radiographs is subjective and prone to observer error. Studies of peri-implant bone measurements report inter- and intra-observer differences on the order of 0.3–0.5 mm or more [55]. Such variability approaches the minimal clinically important change (≈0.2 mm/year of bone loss) and thus can mask early pathological bone loss or falsely suggest stability [55].
• Patient-related and workflow barriers: Practical constraints also limit radiographic evaluation. CBCT imparts a higher radiation dose than conventional films, so its use must be judicious. In orthodontic patients providers reserving CBCT for cases where the added diagnostic benefit justifies the dose [49, 56] Even when CBCT is indicated (e.g. implant in a densely crowded area), obtaining a scan requires additional chair time and expense, and not all orthodontic offices have easy access to in-house CBCT [57].
• Orthodontic appliances themselves can complicate imaging: brackets and wires may need to be removed or repositioned to avoid artifact on a CBCT scan. Patient movement is another issue; children or anxious patients may be less compliant during the longer acquisition time of a 3D scan [58]. Workflow-wise, routine implant planning (especially for mini-implants) still often relies on quick 2D radiographs for efficiency [59]. Finally, since TADs are temporary, clinicians may not schedule multiple follow-up radiographs specifically to assess osseointegration, unlike permanent implants, which can delay detection of complications until clinical symptoms appear [60].
Emerging Technologies and Future Directions
AI and Machine Learning
Deep learning algorithms can now automatically analyze dental radiographs and CBCT scans to detect subtle changes around implants. The performance of the AI models demonstrated considerable variability [61]. Reported overall accuracy ranged from 61.0% to 94.74%, while precision values varied substantially, from as low as 0.63% to as high as 100%. Sensitivity values ranged between 67.0% and 94.44%, whereas specificity ranged from 87.0% to 100% [62]. Integration of AI into clinical workflows also requires standardized datasets and robust validation to ensure consistent performance in routine orthodontic practice [61].
Micro-computed Tomography (micro-CT)
Micro-CT has emerged as a pivotal imaging modality in dental implantology, offering unparalleled insights into bone microarchitecture and implant integration [63] Figure-1 shows the schematic illustration of micro-CT technique [64].
Its high-resolution, three-dimensional imaging capabilities enable detailed assessments of osseointegration, bone volume, and trabecular morphology, which are critical factors for implant success [65]. Recent studies have demonstrated that micro-CT provides superior spatial resolution compared to conventional imaging techniques, such as CBCT and intraoral radiography [63].
This enhanced resolution allows for precise quantification of bone volume fraction (BV/TV), trabecular thickness (Tb.Th), and bone-implant contact (BIC), facilitating a comprehensive evaluation of implant stability and osseointegration [66]. For instance, a systematic review highlighted that micro-CT enables accurate measurements of bone microstructure parameters, which are essential for predicting implant success and longevity [63].
Despite its advantages, the clinical application of micro-CT is limited due to factors such as high radiation exposure, cost, and the necessity for ex vivo analysis [67].
Consequently, its use is predominantly confined to preclinical studies and in vitro assessments [68]. In summary, while micro-CT currently serves as a valuable tool in research settings for evaluating implant stability and osseointegration, further technological advancements are necessary to overcome existing barriers to its routine clinical use [63].
Spectral and Photon-counting CT Imaging
Energy-resolved imaging is an emerging frontier for implant evaluation. Dual-energy CT or CBCT (DECT/DE-CBCT) and photon-counting CT (PCCT) exploit X-ray spectra to improve material discrimination. In phantom studies, dual-energy CBCT has been shown to generate virtual monoenergetic images that significantly reduce metal-induced artifacts around dental implants [69]. Figure-2 illustrate the photon-counting CT Imaging proceeding.
More recently, clinical photon-counting CT scanners (with energy-resolving detectors) have demonstrated sub-millimeter spatial resolution (down to ~100 µm) and inherently reduced metal artifacts when reconstructing images at high virtual energies [69, 70]. For instance, photon-counting CT combined with iterative reconstruction achieved crisper implant detail and higher contrast than conventional CBCT [70] These spectral techniques could, in principle, provide more accurate bone density and composition measurements around orthodontic anchorage implants[71, 72]. Ongoing research is evaluating whether specialized dental CBCT devices can incorporate spectral filtration or energy discrimination in a dose-efficient manner.
Advanced Artifact Reduction and Image Quality Enhancement
Metal artifacts remain a major limitation of radiographic implant evaluation. Recent innovations focus on improved reconstruction and post-processing. Traditional iterative metal-artifact reduction (MAR) algorithms (e.g., projection interpolation) have been supplemented by AI-based denoising [73]. Radiologists in that study also rated the AI-filtered CBCT images as having much lower artifact severity. In practical terms, such noise-reduction could help clinicians better visualize the bone–implant interface despite the presence of brackets or retainers. Additionally, spectral imaging techniques inherently combat artifacts: virtual monoenergetic reconstructions (available in dual-energy or photon-counting CT) reduced beam-hardening around implants [71, 72]. Overall, these advances suggest that future CBCT systems may deliver much cleaner images around metal. Yet, clinical deployment will require integration of vendor-specific software and validation that bone measurement accuracy is not altered by the artifact correction.
Radiomics and Quantitative Image Biomarkers
Radiomics, the high-throughput extraction of quantitative image features, offers a data-driven route to characterize peri-implant bone beyond visual assessment. In dental research, radiomic texture analysis has been used to cluster bone quality.
Troiano et al. [74] demonstrated that unsupervised clustering of CBCT-derived radiomic features from edentulous ridges yielded reproducible stratification of bone types, whereas traditional Lekholm–Zarb qualitative classification showed poor inter-observer agreement [74].
This suggests radiomics could provide an objective metric of bone density or structure relevant to implant stability. Similarly, machine learning models combining CBCT intensity patterns with other clinical data have been proposed to predict insertion torque or resonance frequency of implants. Such quantitative approaches may eventually allow radiographs to serve as “biosensors” of osseointegration [61]. Standardization of acquisition protocols and feature pipelines will be critical before radiomic biomarkers can be clinically adopted for orthodontic implants.
Functional and Dynamic Radiographic Techniques
Beyond static imaging, novel “functional” radiographic methods are being explored. Dynamic digital radiography (DDR), high-frame-rate X-ray sequences, has recently been used in spine and joint imaging to visualize motion or stability under movement [75]. In theory, a similar approach could monitor micro-motion of orthodontic implants under load or capture real-time bone remodeling during orthodontic force application [75]. Another concept is four-dimensional (4D) CBCT, capturing volumetric sequences (e.g., multiple low-dose CBCT over time) to observe bone changes, but radiation dose currently prohibits routine use [76]. To date, these dynamic methods remain experimental; no clinical studies have yet applied DDR or time-resolved CBCT specifically to orthodontic anchorage implants. Nonetheless, future research may adapt low-dose cine-radiography or combine ultrasound with X-ray sensors to provide real-time feedback on implant stability and bone response [77].
Conclusion
Radiographic evaluation remains essential in assessing implant stability and osseointegration among adult orthodontic patients, providing indispensable diagnostic insights that guide clinical decision-making. While conventional imaging techniques such as periapical and panoramic radiographs continue to be routinely used due to their accessibility and low radiation exposure, CBCT has significantly advanced clinical capability by providing comprehensive three-dimensional analysis of bone structures around implants. Nevertheless, inherent limitations such as metal artifacts, diagnostic variability, and patient-specific workflow barriers persist, highlighting the need for continual refinement.
Emerging imaging technologies and innovative analytical techniques such as artificial intelligence-driven diagnostics, spectral and photon-counting CT, advanced artifact-reduction algorithms, and radiomics are poised to overcome many current challenges. These promising methods have demonstrated superior sensitivity, artifact reduction, and quantitative capability, potentially allowing earlier detection of peri-implant bone changes and more precise implant stability assessments. Despite the exciting possibilities offered by these advancements, widespread clinical adoption requires further validation through robust prospective trials and technological standardization.
Future research should focus on rigorous clinical testing and cross-platform validation of these novel imaging methodologies to fully realize their potential benefits. Ultimately, integrating these advanced technologies into routine orthodontic and implant dentistry practices will enhance diagnostic accuracy, optimize patient outcomes, and drive significant progress in managing implant stability and osseointegration
.
Conflict of Interest
None.
|
Radiographic Evaluation of Implant Stability and Osseointegration |
Rostamzadeh S, et al. |
|
GMJ.2025;14:e3799 www.gmj.ir |
3 |
|
Rostamzadeh S, et al. |
Radiographic Evaluation of Implant Stability and Osseointegration |
|
4 |
GMJ.2025;14:e3799 www.gmj.ir |
Table 1. Common Factors Affecting Implant Stability
|
Factor |
Influence on Stability |
Clinical Consideration |
|
Bone Quality |
Higher density bone increases primary stability. |
Preoperative assessment (CBCT), potential bone grafting for low-density bone. |
|
Bone Quantity |
Sufficient bone volume ensures better implant anchorage. |
Ridge augmentation or sinus lift may be required in atrophic ridges. |
|
Implant Design |
Thread design and surface roughness enhance mechanical stability. |
Selection based on patient-specific bone conditions. |
|
Implant Length |
Longer implants provide greater surface area for osseointegration. |
Avoid excessive length near anatomical structures (e.g., nerves, sinuses). |
|
Implant Diameter |
Wider implants distribute occlusal forces better. |
Limited by available bone width; risk of cortical bone resorption. |
|
Surgical Technique |
Precise osteotomy and insertion torque affect primary stability. |
Minimize trauma, consider guided surgery for precision. |
|
Immediate vs. Delayed Loading |
Immediate loading can reduce stability if not well-planned. |
Assess primary stability; delay loading if needed. |
|
Occlusal Forces |
Excessive loading can cause micro-movements and implant failure. |
Proper occlusal adjustments, use of splints if necessary. |
|
Host Factors (Systemic Conditions) |
Conditions like osteoporosis or diabetes may impair osseointegration. |
Preoperative screening and medical management before surgery. |
|
Smoking & Medications |
Smoking and certain drugs (e.g., bisphosphonates) reduce bone healing. |
Encourage smoking cessation; review patient medication history. |
|
Radiographic Evaluation of Implant Stability and Osseointegration |
Rostamzadeh S, et al. |
|
GMJ.2025;14:e3799 www.gmj.ir |
5 |
Table 2. Radiographic Techniques for Implant Stability
|
Imaging Modality |
Primary Use |
Strengths |
Limitations |
Clinical Applicability |
|
Periapical Radiography |
Monitoring crestal bone and implant threads |
High spatial resolution; low cost; low radiation |
2D only; distortion and superimposition; no buccolingual detail |
Routine follow-up; baseline and longitudinal records |
|
Panoramic Radiography |
Full arch overview |
Broad coverage; quick; useful for planning |
Distortion; low resolution; limited detail near implant sites |
Initial evaluation; surgical planning context |
|
CBCT |
3D assessment of peri-implant bone |
Volumetric analysis; accurate bone thickness and density estimation |
Metal artifacts; variable dose; cost; limited HU calibration |
Pre-surgical planning and postoperative evaluation |
|
Micro-CT |
Research-level assessment of osseointegration |
Ultra-high resolution; 3D evaluation of bone-implant interface |
High radiation; ex vivo only; expensive |
Preclinical studies; experimental models |
|
Dynamic Digital Radiography (DDR) |
Functional assessment (experimental) |
Potential to monitor micro-motion and bone remodeling under load |
Still investigational; not yet validated for dental implants |
Future application in real-time implant stability testing |
|
Spectral/Photon-Counting CT |
Artifact reduction; bone quality mapping |
Reduces metal artifacts; high-resolution and contrast |
High cost; limited clinical availability in dentistry |
Emerging; promising for enhanced peri-implant evaluation |
|
Rostamzadeh S, et al. |
Radiographic Evaluation of Implant Stability and Osseointegration |
|
6 |
GMJ.2025;14:e3799 www.gmj.ir |
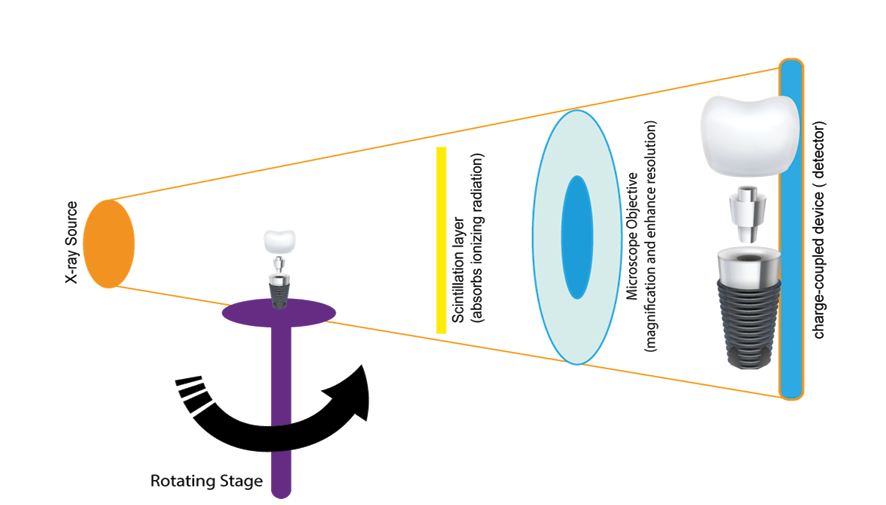
Figure 1. schematic illustration of micro-CT technique
|
Radiographic Evaluation of Implant Stability and Osseointegration |
Rostamzadeh S, et al. |
|
GMJ.2025;14:e3799 www.gmj.ir |
7 |

Figure 2. photon-counting CT Imaging proceeding
|
Rostamzadeh S, et al. |
Radiographic Evaluation of Implant Stability and Osseointegration |
|
8 |
GMJ.2025;14:e3799 www.gmj.ir |
|
Radiographic Evaluation of Implant Stability and Osseointegration |
Rostamzadeh S, et al. |
|
GMJ.2025;14:e3799 www.gmj.ir |
9 |
|
Rostamzadeh S, et al. |
Radiographic Evaluation of Implant Stability and Osseointegration |
|
10 |
GMJ.2025;14:e3799 www.gmj.ir |
|
References |
|
Radiographic Evaluation of Implant Stability and Osseointegration |
Rostamzadeh S, et al. |
|
GMJ.2025;14:e3799 www.gmj.ir |
11 |
|
Rostamzadeh S, et al. |
Radiographic Evaluation of Implant Stability and Osseointegration |
|
12 |
GMJ.2025;14:e3799 www.gmj.ir |
|
Radiographic Evaluation of Implant Stability and Osseointegration |
Rostamzadeh S, et al. |
|
GMJ.2025;14:e3799 www.gmj.ir |
13 |