Gallic Acid Alleviates Injury of Intestine Induced by Escherichia Coli: Protective Role of Metalloproteinase and Antioxidants on Small Intestine In-vivo
Gallic Acid Effects on Escherichia Coli Induced Intestine Injury
DOI:
https://doi.org/10.31661/gmj.v13i.3375Keywords:
Escherichia coli; Gallic acid; anti-inflammatory; antioxidant; MetalloproteinaseAbstract
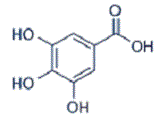
Background: Escherichia coli (E. coli) is a common pathogen that can cause significant morbidity and mortality in hospitalized patients. The aim of this study was to investigate the effects of gallic acid (GA) on a mice infected with of E. coli enteritis and evaluate the serum levels of interleukin-6 (IL-6) and matrix metalloproteinase (MMP)-9, as well as any histopathological changes before and after exposure. Materials and Methods: Forty Swiss male mice were divided into four groups: Group I (negative control), Group II (received oral GA, 80 mg/kg/b.wt), Group III (orally inoculated with E. coli, 1×107 CFU, for four days), and Group IV (received oral GA, 80 mg/kg/b.wt, for 10 days after E. coli inoculation). Serum was collected to assess IL-6 and MMP-9 levels. Intestinal samples were examined for antioxidant parameters, including superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase. Histopathology and immunohistochemistry were performed. Results: Group III exhibited significantly higher IL-6 and MMP-9 levels compared to the other groups (P<0.001). Antioxidant activity in the intestine, measured by SOD and GSH-Px, was lower in Group III compared to Group I. Conversely, Group IV showed significant improvements in biochemical, histopathological, and immunohistochemical outcomes, alongside reduced intestinal damage caused by E. coli. Conclusion: This study demonstrates that E. coli infection in mice increases IL-6 and MMP-9 levels while decreasing intestinal antioxidants. Concurrent administration of GA significantly improves outcomes, suggesting its potential as a therapeutic remedy for E. coli-induced intestinal damage. Furthers research is imperative to determine the underlying pathways by which GA exerts its beneficial outcomes.
References
Qadri F, Svennerholm AM, Faruque ASG, et al. Enterotoxigenic Escherichia coli in Developing Countries: Epidemiology, Microbiology, Clinical Features, Treatment, and Prevention. Clin Microbiol Rev. 2005; 18:465-483
https://doi.org/10.1128/CMR.18.3.465-483.2005
PMid:16020685 PMCid:PMC1195967
World Health Organization. Future directions for research on enterotoxigenic Escherichia coli vaccines for developing countries. Wkly Epidemiol Rec. 2006; 81:97-104.
Ren W, Yin J, Duan J, et al. Mouse intestinal innate immune responses altered by enterotoxigenic Escherichia coli (ETEC) infection. Microbes Infect. 2014; 16:954-961.
https://doi.org/10.1016/j.micinf.2014.09.005
PMid:25267358
Peng XP, Ding W, Ma JM, et al. Effect of Escherichia coli Infection on Metabolism of Dietary Protein in Intestine. Curr Protein Pept Sci. 2020; 21(8):772-776.
https://doi.org/10.2174/1389203720666191113144049
PMid:31724511
Pancu DF, Scurtu A, Macasoi IG, et al. Antibiotics: Conventional Therapy and Natural Compounds with Antibacterial Activity-A Pharmaco-Toxicological Screening. Antibiotics (Basel). 2021; 10(4):401.
https://doi.org/10.3390/antibiotics10040401
PMid:33917092 PMCid:PMC8067816
Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999 Feb;1(2):125-9.
https://doi.org/10.1016/S1286-4579(99)80003-3
PMid:10594976
Chauhan A, Pandey V, Chacko KM, Khandal RK. Antibacterial activity of raw and processed honey. Electron J Biol. 2010;5:58-66. [Google Scholar]
Sanchez-Villamil JI, Navarro-Garcia F, Castillo-Romero A, Gutierrez-Gutierrez F, Tapia D, Tapia-Pastrana G. Curcumin Blocks Cytotoxicity of Enteroaggregative and Enteropathogenic Escherichia coli by Blocking Pet and EspC Proteolytic Release From Bacterial Outer Membrane. Front Cell Infect Microbiol. 2019 Sep 25;9:334.
https://doi.org/10.3389/fcimb.2019.00334
PMid:31681620 PMCid:PMC6798032
Yang K, Zhang L, et al. Impact of Gallic Acid on Gut Health: Focus on the Gut Microbiome, Immune Response, and Mechanisms of Action. Front Immunol. 2020; 11:580208.
https://doi.org/10.3389/fimmu.2020.580208
PMid:33042163 PMCid:PMC7525003
Kahkeshani N, Farzaei F, Fotouhi M, et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran J Basic Med Sci. 2019; 22(3):225-237.
Tian Q, Wei S, Su H, et al. Bactericidal activity of gallic acid against multi-drug resistance Escherichia coli. Microbial Pathogenesis. 2022; 173:105824.
https://doi.org/10.1016/j.micpath.2022.105824
PMid:36243382
Hyun KH, Gil KC, Kim SG, et al. Delphinidin Chloride and its Hydrolytic Metabolite Gallic Acid Promote Differentiation of Regulatory T Cells and Have an Anti-Inflammatory Effect on the Allograft Model. J Food Sci. 2019; 84:920-30.
https://doi.org/10.1111/1750-3841.14490
PMid:30977922
BenSaad LA, Kim KH, Quah CC, et al. Anti-Inflammatory Potential of Ellagic Acid, Gallic Acid and Punicalagin A&B Isolated from Punica Granatum. BMC Complement Altern Med. 2017; 17:47.
https://doi.org/10.1186/s12906-017-1555-0
PMid:28088220 PMCid:PMC5237561
Percie du Sert N, Ahluwalia A, Alam S, et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020 Jul 14;18(7):e3000411.
https://doi.org/10.1371/journal.pbio.3000411
PMid:32663221 PMCid:PMC7360025
Wang Y, Xie M, Ma G, et al. The Antioxidant and Antimicrobial Activities of Different Phenolic Acids Grafted onto Chitosan. Carbohyd Polym. 2019; 225:115238.
https://doi.org/10.1016/j.carbpol.2019.115238
PMid:31521271
Kehl SC. Role of the laboratory in the diagnosis of enterohemorrhagic Escherichia coli infections. J Clin Microbiol. 2002; 40(8):2711-5.
https://doi.org/10.1128/JCM.40.8.2711-2715.2002
PMid:12149318 PMCid:PMC120634
Raj J, Chandra M, Dogra TD, et al. Determination of median lethal dose of combination of endosulfan and cypermethrin in wistar rat. Toxicol Int. 2013; 20(1):1-5.
https://doi.org/10.4103/0971-6580.111531
PMid:23833430 PMCid:PMC3702116
Suresh K. An overview of randomization techniques: An unbiased assessment of outcome in clinical research. Journal of Human Reproductive Sciences. 2011; 4(1): 8-11.
https://doi.org/10.4103/0974-1208.82352
PMid:21772732 PMCid:PMC3136079
El-Naaa M, El-Refaei M, Nasif W, et al. In-vivo antioxidant and anti-inflammatory activity of, rosiglitazone, a peroxisome proliferator-activated receptor-gamma (PPAR-γ) agonists in animal model of bronchial asthma. J Pharm Pharmacol. 2015; 67:1421-1430.
https://doi.org/10.1111/jphp.12445
PMid:26099551
El-Refaei MF, El-Naa MM. Inhibitory effect of caffeic acid phenethyl ester on mice bearing tumor involving angiostatic and apoptotic activities. ChemBiol Interact. 2010; 36(4):383-4.
https://doi.org/10.1016/j.cbi.2010.04.019
PMid:20433813
Nishikimi M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazinemethosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972; 46: 849-854.
https://doi.org/10.1016/S0006-291X(72)80218-3
PMid:4400444
Prins G, Loose J. Glutathione In: Yunis JJ, ed. Biochemical Methods in Red Cell Genetics. New York: Academic Press. 1969; 126-129.
Clairborne A. Catalase activity In. Greenwald RA, ed CRC Handbook of Methods for Oxygen Radical Research: Boca Raton, FL: CRC Press; 1985.
Khan HA, Ibrahim KE, Alrashood ST, et al. Immunohistochemistry of IL-1β, IL-6 and TNF-α in spleens of mice treated with gold nanoparticles. Saudi J Biol Sci. 2020; 27(4):1163-1168.
https://doi.org/10.1016/j.sjbs.2020.01.025
PMid:32256179 PMCid:PMC7105655
Luna LG. Manual of histological staining methods of the forces institute of pathology 3rd edition. McGraw: Hillbook, New York; 1968.
Gibson-Corley KN, Olivier AK, Meyerholz DK. Principles for valid histopathologic scoring in research. Vet Pathol. 2013; 50(6):1007-15.
https://doi.org/10.1177/0300985813485099
PMid:23558974 PMCid:PMC3795863
Faul F, Erdfelder E, Lang AG, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007; 39(2):175-91.
https://doi.org/10.3758/BF03193146
PMid:17695343
Crowe AR, Yue W. Semi-quantitative Determination of Protein Expression using Immunohistochemistry Staining and Analysis: An Integrated Protocol. Bio Protoc. 2019;9(24):e3465.
https://doi.org/10.21769/BioProtoc.3465
Citation in APA style. Microsoft Corporation. Citation in Vancouver style: Microsoft Corporation Microsoft Excel; Available from: https://office.microsoft.com/excel.
Hazra A, Gogtay N. Biostatistics Series Module 3: Comparing Groups: Numerical Variables. Indian J Dermatol. 2016; 61(3):251-60.
https://doi.org/10.4103/0019-5154.182416
PMid:27293244 PMCid:PMC4885176
Jia ZF, Chen A, Bao F, et al. Effect of Nisin on Microbiome-Brain-Gut Axis Neurochemicals by Escherichia coli -Induced Diarrhea in Mice. Microb Pathog. 2018;119:65-71.
https://doi.org/10.1016/j.micpath.2018.04.005
PMid:29649517
Qin J, Li R, Raes J, et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature. 2010; 464:59-65.
https://doi.org/10.1038/nature08821
PMid:20203603 PMCid:PMC3779803
Pabst R, Russell MW, Brandtzaeg P. Tissue Distribution of Lymphocytes and Plasma Cells and the Role of the Gut. Trends Immunol. 2008; 29:206-208.
https://doi.org/10.1016/j.it.2008.02.006
PMid:18394963
Hiippala K, Jouhten H, Ronkainen A, et al. The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients. 2018; 10:988
https://doi.org/10.3390/nu10080988
PMid:30060606 PMCid:PMC6116138
Jacobi SK, Jack O. Nutritional Factors Influencing Intestinal Health of the Neonate. Adv Nutr. 2012; 3:687-696.
https://doi.org/10.3945/an.112.002683
PMid:22983847 PMCid:PMC3648750
Ching CB, Gupta S, Li B, Cortado H, Mayne N, Jackson AR, McHugh KM, Becknell B. Interleukin-6/Stat3 signaling has an essential role in the host antimicrobial response to urinary tract infection. Kidney Int. 2018; 93(6):1320-1329.
https://doi.org/10.1016/j.kint.2017.12.006
PMid:29475562 PMCid:PMC5967986
He L, Wang C, Simujide H, et al. Effect of Early Pathogenic Escherichia coli Infection on the Intestinal Barrier and Immune Function in Newborn Calves. Front Cell Infect Microbiol. 2022; 12:818276.
https://doi.org/10.3389/fcimb.2022.818276
PMid:35265533 PMCid:PMC8900010
Fayyaz I, Zahoor MA, Shahid M, et al. Effect of Lactobacillus casei on serum interleukins following enteropathogenic E coli infection in experimental rabbits. Pak J Pharm Sci. 2018; 31(5):2131-2136.
Khmaladze I, Österlund C, Smiljanic S, et al. A novel multifunctional skin care formulation with a unique blend of antipollution, brightening and antiaging active complexes. J Cosmet Dermatol. 2020; 19(6):1415-1425.
https://doi.org/10.1111/jocd.13176
PMid:31584241
Liu S, Li J, Feng LH. Gallic acid regulates immune response in a mouse model of rheumatoid arthritis. Immun Inflamm Dis. 2023 Feb;11(2):e782.
https://doi.org/10.1002/iid3.782
PMid:36840490 PMCid:PMC9933205
Lee HS, Kim WJ. The Role of Matrix Metalloproteinase in Inflammation with a Focus on Infectious Diseases. Int J Mol Sci. 2022; 23(18):10546.
https://doi.org/10.3390/ijms231810546
PMid:36142454 PMCid:PMC9500641
Bellioglu YE, Coskun YZM, Ersoz M, et al. Effects of gallic acid on expressions of MMP-2 and MMP-9 through the pathway of p38/JNK in C6 glioma cells. Pak J Pharm Sci. 2023; 36(1):59-66.
Tian Q, Wei S, Su H, et al. Bactericidal activity of gallic acid against multi-drug resistance Escherichia coli. Microb Pathog. 2022; 173:105824.
https://doi.org/10.1016/j.micpath.2022.105824
PMid:36243382
Saxena P, Selvaraj K, Khare SK, et al. Superoxide dismutase as multipotent therapeutic antioxidant enzyme: Role in human diseases. Biotechnol Lett. 2022; 44(1):1-22.
https://doi.org/10.1007/s10529-021-03200-3
PMid:34734354
Lin X, Bai D, Wei Z, et al. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS One. 2019; 14(5):e0216711.
https://doi.org/10.1371/journal.pone.0216711
PMid:31112588 PMCid:PMC6528975
Esmaeilzadeh M, Heidarian E, Shaghaghi M, et al. Gallic acid mitigates diclofenac-induced liver toxicity by modulating oxidative stress and suppressing IL-1β gene expression in male rats. Pharm Biol. 2020; 58(1):590-596.
https://doi.org/10.1080/13880209.2020.1777169
PMid:32633182 PMCid:PMC7470116
Santos A, Finlay BB. Bringing down the host: enteropathogenic and enterohaemorrhagic Escherichia coli effector-mediated subversion of host innate immune pathways. Cell Microbiol. 2015;17(3):318-332.
https://doi.org/10.1111/cmi.12412
PMid:25588886
Bai J, Zhang Y, Tang C, et al. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomedicine & Pharmacotherapy. 2021;133:110985.
https://doi.org/10.1016/j.biopha.2020.110985
PMid:33212373
Zheng L, Duan SL, Dai YC, et al. Role of adherent invasive Escherichia coli in pathogenesis of inflammatory bowel disease. World J Clin Cases. 2022; 10(32):11671-11689.
https://doi.org/10.12998/wjcc.v10.i32.11671
PMid:36405271 PMCid:PMC9669839
Brauner A, Söderhäll M, Jacobson SH, et al. Escherichia coli-induced expression of IL-1 alpha, IL-1 beta, IL-6 and IL-8 in normal human renal tubular epithelial cells. Clin Exp Immunol. 2001; 124(3):423-8.
https://doi.org/10.1046/j.1365-2249.2001.01533.x
PMid:11472403 PMCid:PMC1906084
Albrecht LJ, Tauber SC, Merres J, et al. Lack of Proinflammatory Cytokine Interleukin-6 or Tumor Necrosis Factor Receptor-1 Results in a Failure of the Innate Immune Response after Bacterial Meningitis. Mediators Inflamm. 2016; 2016:7678542.
https://doi.org/10.1155/2016/7678542
PMid:27057100 PMCid:PMC4749820
Seo CS, Jeong SJ, Yoo SR, Lee NR, Shin HK. Quantitative Analysis and In vitro Anti-inflammatory Effects of Gallic Acid, Ellagic Acid, and Quercetin from Radix Sanguisorbae. Pharmacogn Mag. 2016;12(46):104-8.
https://doi.org/10.4103/0973-1296.177908
PMid:27076745 PMCid:PMC4809163
Pandurangan AK, Mohebali N, Esa NM, et al. Gallic acid suppresses inflammation in dextran sodium sulfate-induced colitis in mice: possible mechanisms. Int Immunopharmacol. 2015; 28:1034-43.
https://doi.org/10.1016/j.intimp.2015.08.019
PMid:26319951
Kim H, Venancio VP, Fang C, et al. Mango (Mangifera indica L.) polyphenols reduce IL-8, GRO, and GM-SCF plasma levels and increase Lactobacillus species in a pilot study in patients with inflammatory bowel disease. Nutr Res. 2020; 75:85-94.
https://doi.org/10.1016/j.nutres.2020.01.002
PMid:32109839
Savkovic SD, Villanueva J, Turner JR, et al. Mouse model of enteropathogenic Escherichia coli infection. Infect Immun. 2005; 73(2):1161-70.
https://doi.org/10.1128/IAI.73.2.1161-1170.2005
PMid:15664959 PMCid:PMC546940
Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004; 2(2):123-40.
https://doi.org/10.1038/nrmicro818
PMid:15040260
Lin TH, Wu CC, Tseng CY, et al. Effects of gallic acid on capsular polysaccharide biosynthesis in Klebsiella pneumoniae. J Microbiol Immunol Infect. 2022; 55(6):1255-1262.
https://doi.org/10.1016/j.jmii.2021.07.002
PMid:34326026
Lee J, Choi KH, Min J, et al. Functionalized ZnO Nanoparticles with Gallic Acid for Antioxidant and Antibacterial Activity against Methicillin-Resistant S aureus. Nanomaterials (Basel). 2017; 7(11):365.
https://doi.org/10.3390/nano7110365
PMid:29099064 PMCid:PMC5707582
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Galen Medical Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.







