An Investigation of the Effects of Formononetin on Hypothalamic Gonadotropin-releasing Hormone, Kisspeptin and Tachykinin 2 Gene Expression in Rats
DOI:
https://doi.org/10.31661/gmj.v14i.3549Keywords:
Formononetin; Kisspeptin; Neurokinin B; GnRHAbstract
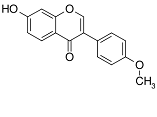
Background: Red clover and its main derivative, formononetin, belong to the phytoestrogens. They are clinically used to alleviate mood disorders, anxiety, and hot flashes. Formononetin may interfere with the reproductive axis due to its estrogenic potency and its ability to bind estrogen receptors. To find some molecular mechanisms mediating the effects of formononetin on the hypothalamus-pituitary-gonadal (HPG) axis, this research aimed to investigate the effects of formononetin on the hypothalamic mRNA levels of gonadotropin-releasing hormone (Gnrh), kisspeptin1(Kiss1) and tachykinin 2 (Tac2).
Materials and Methods: Fifteen male Wistar rats weighing 200±10 g were divided into three groups (n=5). Group 1 as the control group, received saline. Groups 2 and 3 received 20 and 40 µg of formononetin via the third cerebral ventricle. The hypothalamic samples were dissected. The Gnrh, Kiss1 and Tac2 gene expression was measured by real-time PCR. Results: Injection of 20 µg formononetin did not significantly decrease the mRNA levels of Gnrh and Tac2 compared to the control group. However, injection of 40 µg formononetin significantly reduced the mRNA levels of Gnrh and Tac2 compared to the control group. Injection of 20 and 40 µg formononetin, significantly declined the mRNA levels of Kiss1 compared to the control group.
Conclusion: Present results indicated that formononetin may be involved in the regulation of the reproductive axis via reducing the activity of hypothalamic GnRH neurons and downregulation of the kisspeptin and neurokinin B signaling pathways upstream of GnRH neurons.
References
Acevedo-Rodriguez A, Kauffman A, Cherrington B, Borges C, Roepke TA, Laconi M. Emerging insights into hypothalamic‐pituitary-gonadal axis regulation and interaction with stress signalling. J Neuroendocrinol. 2018;30(10):e12590.
https://doi.org/10.1111/jne.12590
PMid:29524268 PMCid:PMC6129417
Chatterjee A, Rajikin MH, Chatterjee R, Ghosh S. Stress and how it affects reproduction. Biomed Res. 2006;17:1-6.
Morales Ramírez M, Vargas Estrada D, Juárez Rodríguez I, Pérez-Rivero JJ, Sierra Reséndiz A, Flores González HF et al. Effects of phytoestrogens on the reproductive physiology of productive species Review. Rev Mex Cienc Pecu. 2022;13(3):803-29.
https://doi.org/10.22319/rmcp.v13i3.5878
Szeliga A, Czyzyk A, Podfigurna A, Genazzani AR, Genazzani AD, Meczekalski B. The role of kisspeptin/neurokinin B/dynorphin neurons in pathomechanism of vasomotor symptoms in postmenopausal women: from physiology to potential therapeutic applications. Gynecol Endocrinol. 2018;34(11):913-9.
https://doi.org/10.1080/09513590.2018.1480711
PMid:29902942
Uenoyama Y, Nagae M, Tsuchida H, Inoue N, Tsukamura H. Role of KNDy neurons expressing kisspeptin, neurokinin B, and dynorphin A as a GnRH pulse generator controlling mammalian reproduction. Front Endocrinol. 2021;12:724632.
https://doi.org/10.3389/fendo.2021.724632
PMid:34566891 PMCid:PMC8458932
Khazali H, Mahmoudi F, Janahmadi M. Hypothalamic KiSS1/GPR54 gene expressions and luteinizing hormone plasma secretion in morphine treated male rats. Int J Fertil Steril. 2018;12(3):223.
Clarke H, Dhillo WS, Jayasena CN. Comprehensive review on kisspeptin and its role in reproductive disorders. Endocrinol Metab. 2015;30(2):124-41.
https://doi.org/10.3803/EnM.2015.30.2.124
PMid:26194072 PMCid:PMC4508256
Mahmoudi F, Khazali H, Janahmadi M. Morphine attenuates testosterone response to central injection of kisspeptin in male rats. Int J Fertil Steril. 2014;8(2):215.
Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin B and the hypothalamic regulation of reproduction. Brain RES. 2010;1364:116-28.
https://doi.org/10.1016/j.brainres.2010.08.059
PMid:20800582 PMCid:PMC2992576
Anderson R, Skorupskaite K, Sassarini J. The neurokinin B pathway in the treatment of menopausal hot flushes. Climacteric. 2019;22(1):51-4.
https://doi.org/10.1080/13697137.2018.1540564
PMid:30572747
Young J, George JT, Tello JA, Francou B, Bouligand J, Guiochon-Mantel A et al. Kisspeptin restores pulsatile LH secretion in patients with neurokinin B signaling deficiencies: physiological, pathophysiological and therapeutic implications. Neuroendocrinology. 2013;97(2):193-202.
https://doi.org/10.1159/000336376
PMid:22377698 PMCid:PMC3902960
Akbaribazm M, Khazaei F, Naseri L, Pazhouhi M, Zamanian M, Khazaei M. Pharmacological and therapeutic properties of the Red Clover (Trifolium pratense L) an overview of the new findings. J Tradit Chin Med. 2021;41(4):642-649.
Ding M, Bao Y, Liang H, Zhang X, Li B, Yang R et al. Potential mechanisms of formononetin against inflammation and oxidative stress: a review. Front Pharmacol. 2024;15:1368765.
https://doi.org/10.3389/fphar.2024.1368765
PMid:38799172 PMCid:PMC11116718
Mu H, Bai Y-H, Wang S-T, Zhu Z-M, Zhang Y-W. Research on antioxidant effects and estrogenic effect of formononetin from Trifolium pratense (red clover). Phytomedicine. 2009;16(4):314-9.
https://doi.org/10.1016/j.phymed.2008.07.005
PMid:18757188
Yigit E, Unsal S. Isoflavones obtained from red clover improve both dyslipidemia and menopausal symptoms in menopausal women: a prospective randomized placebo-controlled trial. Climacteric. 2024:1-7.
https://doi.org/10.1080/13697137.2024.2393121
PMid:39254422
Oza MJ, Kulkarni YA. Formononetin treatment in type 2 diabetic rats reduces insulin resistance and hyperglycemia. Front Pharmacol. 2018;9:739.
https://doi.org/10.3389/fphar.2018.00739
PMid:30072892 PMCid:PMC6058024
Wang X-s, Guan S-y, Liu A, Yue J, Hu L-n, Zhang K et al. Anxiolytic effects of Formononetin in an inflammatory pain mouse model. Mol brain. 2019;12:1-12.
https://doi.org/10.1186/s13041-019-0453-4
PMid:30961625 PMCid:PMC6454770
Ghazanfarpour M, Sadeghi R, Roudsari RL, Najmabadi KM, Khadivzadeh T. Effects of red clover on hot flash and circulating hormone concentrations in menopausal women: a systematic review and meta-analysis. Avicenna J Phytomed. 2015;5(6):498.
Paxinos G, Watson C. The rat brain in stereotaxic coordinates. hard cover edition: Elsevier; 2006.
Basirat E, Mahmoudi F, Khazali H. The expression of melanin concentrating hormone and corticotrophin releasing hormone genes in a stress model rats receiving formononetin. Gene, Cell, Tissue. 2025; 12(1):1-6.
https://doi.org/10.5812/gct-150920
McGarvey C, Cates PS, Brooks AN, Swanson IA, Milligan SR, Coen CW et al. Phytoestrogens and gonadotropin-releasing hormone pulse generator activity and pituitary luteinizing hormone release in the rat. Endocrinology. 2001;142(3):1202-8.
https://doi.org/10.1210/endo.142.3.8015
PMid:11181536
Kelly MJ, Rønnekleiv OK. Minireview: neural signaling of estradiol in the hypothalamus. Mol Endocrinol. 2015;29(5):645-57.
https://doi.org/10.1210/me.2014-1397
PMid:25751314 PMCid:PMC4415204
Azcoitia I, Mendez P, Garcia-Segura LM. Aromatase in the human brain. Androg Clin Res Ther. 2021;2(1):189-202.
https://doi.org/10.1089/andro.2021.0007
PMid:35024691 PMCid:PMC8744447
Korani M. Aromatase inhibitors in male: A literature review. Med Clin Práctica. 2023;6(1):100356.
https://doi.org/10.1016/j.mcpsp.2022.100356
Sandini TM, Reis-Silva TM, Moreira N, Bernardi MM, Lebrun I, Spinosa HdS. Effects of isoflavones on behavior, estradiol, glutamate, and GABA levels in intact middle-aged female rats. Nutr Neurosci. 2019;22(11):805-16.
https://doi.org/10.1080/1028415X.2018.1447296
PMid:29514592
Li S, Dang Y, Zhou X, Huang B, Huang X, Zhang Z et al. Formononetin promotes angiogenesis through the estrogen receptor alpha-enhanced ROCK pathway. Sci Rep. 2015;5(1):16815.
https://doi.org/10.1038/srep16815
PMid:26568398 PMCid:PMC4645220
Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, Van Der Saag PT et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998;139(10):4252-63.
https://doi.org/10.1210/endo.139.10.6216
PMid:9751507
Chen Y-M, Wang I-L, Zhu X-Y, Chiu W-C, Chiu Y-S. Red clover isoflavones influence estradiol concentration, exercise performance, and gut microbiota in female mice. Front Nutr. 2021;8:623698.
https://doi.org/10.3389/fnut.2021.623698
PMid:33937304 PMCid:PMC8079722
Waris Gh, Ahmed A, Rukhsar A, Zahra F, A M. Formononetin as promising therapeutic intervention for restoring ovarian, uterine, and hepato renal functions in letrozole- induced polycystic ovarian syndrome sprague dawley. J Popul Ther Clin Pharmacol. 2023;30(19):727-35.
https://doi.org/10.53555/jptcp.v30i19.3749
Qiu J, Rivera HM, Bosch MA, Padilla SL, Stincic TL, Palmiter RD et al. Estrogenic-dependent glutamatergic neurotransmission from kisspeptin neurons governs feeding circuits in females. Elife. 2018;7:e35656.
https://doi.org/10.7554/eLife.35656
PMid:30079889 PMCid:PMC6103748
Nestor CC, Qiu J, Padilla SL, Zhang C, Bosch MA, Fan W et al. Optogenetic stimulation of arcuate nucleus Kiss1 neurons reveals a steroid-dependent glutamatergic input to POMC and AgRP neurons in male mice. Mol Endocrinol. 2016;30(6):630-44.
https://doi.org/10.1210/me.2016-1026
PMid:27093227 PMCid:PMC4884339
Iwata K, Ogata R, Sato M, Matsuda F, Ishii H, Ozawa H. Short-term depletion of plasma estrogen affects hypothalamic kisspeptin-neurokinin B-dynorphin a neurons, gonadotrophs, and pulsatile luteinizing hormone secretion in female rats. Peptides. 2023;160:170929.
https://doi.org/10.1016/j.peptides.2022.170929
PMid:36574861
Morris PG, Herbison AE. Mechanism of arcuate kisspeptin neuron synchronization in acute brain slices from female mice. Endocrinology. 2023;164(12):bqad167.
https://doi.org/10.1210/endocr/bqad167
PMid:37936337 PMCid:PMC10652333
Cavendish RL, de Souza Santos J, Neto RB, Paixão AO, Oliveira JV, de Araujo ED et al. Antinociceptive and anti-inflammatory effects of Brazilian red propolis extract and formononetin in rodents. J Ethnopharmacol. 2015;173:127-33.
https://doi.org/10.1016/j.jep.2015.07.022
PMid:26192808
Tian J, Wang X-Q, Tian Z. Focusing on formononetin: recent perspectives for its neuroprotective potentials. Front Pharmacol. 2022;13:905898.
https://doi.org/10.3389/fphar.2022.905898
PMid:35712702 PMCid:PMC9196267
Yu D, Duan Y, Bao Y, Wei C, An L. Isoflavonoids from Astragalus mongholicus protect PC12 cells from toxicity induced by L-glutamate. J Ethnopharmacol. 2005;98(1-2):89-94.
https://doi.org/10.1016/j.jep.2004.12.027
PMid:15763368
Prague JK, Voliotis M, Clarke S, Comninos AN, Abbara A, Jayasena CN et al. Determining the relationship between hot flushes and LH Pulses in menopausal women using mathematical modeling. J Clin Endocrinol Metab. 2019;104(9):3628-36.
https://doi.org/10.1210/jc.2018-02797
PMid:30985867 PMCid:PMC6637789
Jayasena CN, Comninos AN, Stefanopoulou E, Buckley A, Narayanaswamy S, Izzi-Engbeaya C et al. Neurokinin B administration induces hot flushes in women. Sci Rep. 2015;5(1):8466.
https://doi.org/10.1038/srep08466
PMid:25683060 PMCid:PMC4329553
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Galen Medical Journal

This work is licensed under a Creative Commons Attribution 4.0 International License.







