Preparation and Characterization of Myristoylated Chitosan Nanogel as Carrier of Silibinin for Breast Cancer Therapy
DOI:
https://doi.org/10.31661/gmj.v6i2.822Keywords:
Chitosan, Myristoylated, Silibinin, Nanogel, Breast CancerAbstract
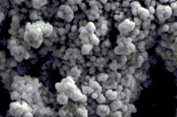
Background: Biopolymer has been known to have compatibility; nontoxic nature and degradation behavior. Chitosan (CS) is being widely used in various biomedical and pharmaceutical applications and serves as a drug carrier. Nanotechnology has emerged as tumor cell target therapy and increase drug bioavailability. One of the most common and important models of cancer in women is breast cancer, which is the fifth most common death reason. Silibinin (SIL) as a flavonolignan, has demonstrated anticancer effects against various human cancer cells, such as breast cancer. Materials and Methods: Myristoylated CS (MCS) nanoparticles were prepared on the base of 9:1 ratio related to CS: Myristate and loaded with SIL, for the first time. Then in vitro loading and releasing capacity of nano drug were evaluated. The nanogel structure and its derivatives were characterized by different biophysical methods. The MCF-7 breast cancer cell line and human umbilical vein endothelial cells (HUVEC) cell lines were incubated with 100, 150, and 200μg/ml of SIL and nanoSIL. Afterward cell cytotoxicity was measured by MTT assay. Lethal dose 50 (LD50) or IC50 was measured by Pharm software. Results: Compared to HUVEC as normal cells, the proliferation of MCF-7 cells were significantly inhibited (P<0.01) by SIL and nano-SIL in a concentration-related manner in defining times (P<0.05). SIL-loaded nanogels were more effective than SIL alone (P<0.01). The mean size of MCS particles was about 20nm. The MCS nanogels were spheral and homogen with a dense surface. The loading efficiency was obtained about 85-95%. Conclusions: It seems the obtained MCS nanogel can play a real and important role as a suitable drug carrier with a high loading capacity to treat cancerous cells with the least side effect. [GMJ.2017;6(2):136-44]