In Vitro Spermatogenesis by Three-dimensional Culture of Spermatogonial Stem Cells on Decellularized Testicular Matrix
DOI:
https://doi.org/10.31661/gmj.v8i.1565Keywords:
Spermatogonial Stem Cells, Decellularization, Testicular Matrix, Proliferation, DiffetentiationAbstract
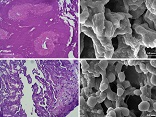
Background: In the males, Spermatogonial Stem Cells (SSCs) contribute to the production of sex cells and fertility. In vitro SSCs culture can operate as an effective strategy for studies on spermatogenesis and male infertility treatment. Cell culture in a three-dimensional (3D) substrate, relative to a two-dimensional substrate (2D), creates better conditions for cell interaction and is closer to in vivo conditions. In the present study, in order to create a 3D matrix substrate, decellularized testicular matrix (DTM) was used to engender optimal conditions for SSCs culture and differentiation. Materials and Methods: After, testicular cells enzymatic extraction from testes of brain-dead donors, the SSCs were proliferated in a specific culture medium for four weeks, and after confirming the identity of the colonies derived from the growth of these cells, they were cultured on a layer of DTM as well as in 2D condition with a differentiated culture medium. In the Sixth week since the initiation of the differentiation culture, the expression of pre meiotic (OCT4 & PLZF), meiotic (SCP3 & BOULE) and post meiotic (CREM & Protamine-2) genes were measured in both groups. Results: The results indicated that the expression of pre meiotic, meiotic and post meiotic genes was significantly higher in the cells cultured on DTM (P ≤ 0.001). Conclusion: SSCs culture in DTM with the creation of ECM and similar conditions with in vivo can be regarded as a way of demonstrating spermatogenesis in vitro, which can be adopted as a treatment modality for male infertility. [GMJ.2019;8:e1565]Â
References
De Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21(6):776-98. Craft I, Bennett V, Nicholson N. Fertilising ability of testicular spermatozoa. Lancet. 1993;342(8875):864. https://doi.org/10.1016/0140-6736(93)92722-6 Marx V. Cell culture: a better brew. Nature; 2013. https://doi.org/10.1038/496253aPMid:23579682 Souza GR, Molina JR, Raphael RM, Ozawa MG, Stark DJ, Levin CS et al. Three-dimensional tissue culture based on magnetic cell levitation. Nat Nanotechnol. 2010;5(4):291. https://doi.org/10.1038/nnano.2010.23PMid:20228788 PMCid:PMC4487889 Pampaloni F, Reynaud EG, Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8(10):839. https://doi.org/10.1038/nrm2236PMid:17684528 Chun T-H, Hotary KB, Sabeh F, Saltiel AR, Allen ED, Weiss SJ. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125(3):577-91. https://doi.org/10.1016/j.cell.2006.02.050PMid:16678100 Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130(4):601-10. https://doi.org/10.1016/j.cell.2007.08.006PMid:17719539 Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA. Spheroid-based drug screen: considerations and practical approach. Nat Protoc. 2009;4(3):309. https://doi.org/10.1038/nprot.2008.226PMid:19214182 Prestwich GD. Simplifying the extracellular matrix for 3-D cell culture and tissue engineering: A pragmatic approach. J Cell Biochem. 2007;101(6):1370-83. https://doi.org/10.1002/jcb.21386PMid:17492655 Baert Y, Goossens E. Preparation of scaffolds from decellularized testicular matrix. Methods Mol Biol. 2017:257-84. https://doi.org/10.1007/7651_2017_29PMid:28456952 Vermeulen M, Del Vento F, de Michele F, Poels J, Wyns C. Development of a cytocompatible scaffold from pig immature testicular tissue allowing human sertoli cell attachment, proliferation and functionality. Int J Mol. Sci.2018;19(1):227. https://doi.org/10.3390/ijms19010227PMid:29329231 PMCid:PMC5796176 Baert Y, Stukenborg J-B, Landreh M, De Kock J, Jörnvall H, Söder O et al. Derivation and characterization of a cytocompatible scaffold from human testis. Hum Reprod.2014;30(2):256-67. https://doi.org/10.1093/humrep/deu330PMid:25505010 Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69(2):612-6. https://doi.org/10.1095/biolreprod.103.017012PMid:12700182 Sadri-Ardekani H, Mizrak SC, van Daalen SK, Korver CM, Roepers-Gajadien HL, Koruji M et al. Propagation of human spermatogonial stem cells in vitro. Jama. 2009;302(19):2127-34. https://doi.org/10.1001/jama.2009.1689PMid:19920237 Lee DR, Kaproth MT, Parks JE. In vitro production of haploid germ cells from fresh or frozen-thawed testicular cells of neonatal bulls. Biol Reprod. 2001;65(3):873-8. https://doi.org/10.1095/biolreprod65.3.873PMid:11514353 Berna G, Leon-Quinto T, Ensenat-Waser R, Montanya E, Martin F, Soria B. Stem cells and diabetes. Biomed Pharmacother. 2001;55(4):206-12. https://doi.org/10.1016/S0753-3322(01)00050-6 Stukenborg JB, Wistuba J, Luetjens CM, Elhija MA, Huleihel M, Lunenfeld E et al. Coculture of spermatogonia with somatic cells in a novel three-dimensional soft-agar-culture-system. J Androl. 2008;29(3):312-29. https://doi.org/10.2164/jandrol.107.002857PMid:18046051 Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A et al. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011;471(7339):504. https://doi.org/10.1038/nature09850PMid:21430778 Gholami K, Pourmand G, Koruji M, Sadighigilani M, Navid S, Izadyar F et al. Efficiency of colony formation and differentiation of human spermatogenic cells in two different culture systems. Reprod Biol. 2018. https://doi.org/10.1016/j.repbio.2018.09.006PMid:30291003 Gholami K, Pourmand G, Koruji M, Ashouri S, Abbasi M. Organ culture of seminiferous tubules using a modified soft agar culture system. Stem Cell Res. Ther. 2018;9(1):249. https://doi.org/10.1186/s13287-018-0997-8PMid:30257723 PMCid:PMC6158910 Khajavi N, Akbari M, Abolhassani F, Dehpour AR, Koruji M, Roudkenar MH. Role of somatic testicular cells during mouse spermatogenesis in three-dimensional collagen gel culture system. Cell J. 2014;16(1):79. Huleihel M, Nourashrafeddin S, Plant TM. Application of three-dimensional culture systems to study mammalian spermatogenesis, with an emphasis on the rhesus monkey (Macaca mulatta). Asian J Androl. 2015;17(6):972. https://doi.org/10.4103/1008-682X.154994PMid:26067870 PMCid:PMC4814948 Lee JH, Kim HJ, Kim H, Lee SJ, Gye MC. In vitro spermatogenesis by three-dimensional culture of rat testicular cells in collagen gel matrix. Biomaterials. 2006;27(14):2845-53. https://doi.org/10.1016/j.biomaterials.2005.12.028PMid:16430959 Orwig KE, Ryu B-Y, Avarbock MR, Brinster RL. Male germ-line stem cell potential is predicted by morphology of cells in neonatal rat testes. Proc Natl Acad Sci U S A. 2002;99(18):11706-11. https://doi.org/10.1073/pnas.182412099PMid:12185252 PMCid:PMC129333 Crapo PM, Tottey S, Slivka PF, Badylak SF. Effects of biologic scaffolds on human stem cells and implications for CNS tissue engineering. Tissue Eng Part A. 2013;20(1-2):313-23. https://doi.org/10.1089/ten.tea.2013.0186PMid:24004192 PMCid:PMC3875189 Crapo PM, Medberry CJ, Reing JE, Tottey S, van der Merwe Y, Jones KE et al. Biologic scaffolds composed of central nervous system extracellular matrix. Biomaterials. 2012;33(13):3539-47. https://doi.org/10.1016/j.biomaterials.2012.01.044PMid:22341938 PMCid:PMC3516286 Navarro-Tableros V, Herrera Sanchez MB, Figureliolini F, Romagnoli R, Tetta C, Camussi G. Recellularization of rat liver scaffolds by human liver stem cells. Tissue Eng Part A. 2015;21(11-12):1929-39. https://doi.org/10.1089/ten.tea.2014.0573PMid:25794768 PMCid:PMC4449720 Cortiella J, Niles J, Cantu A, Brettler A, Pham A, Vargas G et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A. 2010;16(8):2565-80. https://doi.org/10.1089/ten.tea.2009.0730PMid:20408765 Makrigiannakis A, Coukos G, Blaschuk O, Coutifaris C. Follicular Atresia and Luteolysis Evidence of a Role for N-Cadherin. Ann N Y Acad Sci. 2000;900(1):46-55. https://doi.org/10.1111/j.1749-6632.2000.tb06215.xPMid:10818391 Laurie G, Leblond C, Martin G. Localization of type IV collagen, laminin, heparan sulfate proteoglycan, and fibronectin to the basal lamina of basement membranes. Int J Biochem Cell Biol. 1982;95(1):340-4. https://doi.org/10.1083/jcb.95.1.340PMid:6216257 PMCid:PMC2112347 Oğuzkurt P, Kayaselçuk F, Tuncer İ, Alkan M, Hiçsönmez A. Evaluation of extracellular matrix protein composition in sacs associated with undescended testis, hydrocele, inguinal hernia, and peritoneum. Urology. 2007;70(2):346-50. https://doi.org/10.1016/j.urology.2007.03.030PMid:17826504 Wierzbicka-Patynowski I, Schwarzbauer JE. The ins and outs of fibronectin matrix assembly. J Cell Sci. 2003;116(16):3269-76. https://doi.org/10.1242/jcs.00670PMid:12857786 Giudice MG, De Michele F, Poels J, Vermeulen M, Wyns C. Update on fertility restoration from prepubertal spermatogonial stem cells: how far are we from clinical practice? Stem Cell Res. 2017;21:171-7. https://doi.org/10.1016/j.scr.2017.01.009PMid:28174013 Shams A, Eslahi N, Movahedin M, Izadyar F, Asgari H, Koruji M. Future of spermatogonial stem cell culture: application of nanofiber scaffolds. Curr Stem Cell Res Ther. 2017;12(7):544-53. https://doi.org/10.2174/1574888X12666170623095457PMid:28641554 Amsterdam A, Gold RS, Hosokawa K, Yoshida Y, Sasson R, Jung Y et al. Crosstalk among multiple signaling pathways controlling ovarian cell death. Trends Endocrinol Metab. 1999;10(7):255-62. https://doi.org/10.1016/S1043-2760(99)00164-2